Abstract
Objective
The aim of this systematic review was to provide a comprehensive and detailed review of structural and methodological assumptions in model-based cost-effectiveness analyses of systemic metastatic colorectal cancer (mCRC) treatments, and discuss their potential impact on health economic outcome estimates.
Methods
Five databases (EMBASE, MEDLINE, Cochrane Library, Health Technology Assessment and National Health Service Health Economic Evaluation Database) were searched on 26 August 2019 for model-based full health economic evaluations of systemic mCRC treatment using a combination of free-text terms and subject headings. Full-text publications in English were eligible for inclusion if they were published in or after the year 2000. The Consolidated Health Economic Evaluation Reporting Standards checklist was used to assess the reporting quality of included publications. Study selection, appraisal and data extraction were performed by two reviewers independently.
Results
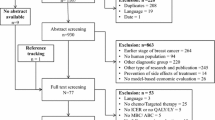
The search yielded 1418 publications, of which 54 were included, representing 51 unique studies. Most studies focused on first-line treatment (n = 29, 57%), followed by third-line treatment (n = 13, 25%). Model structures were health-state driven (n = 27, 53%), treatment driven (n = 19, 37%), or a combination (n = 5, 10%). Cohort-level state-transition modelling (STM) was the most common technique (n = 33, 65%), followed by patient-level STM and partitioned survival analysis (both n = 6, 12%). Only 15 studies (29%) reported some sort of model validation. Health economic outcomes for specific strategies differed substantially between studies. For example, survival following first-line treatment with fluorouracil, leucovorin and oxaliplatin ranged from 1.21 to 7.33 years, with treatment costs ranging from US$8125 to US$126,606.
Conclusions
Model-based cost-effectiveness analyses of systemic mCRC treatments have adopted varied modelling methods and structures, resulting in substantially different outcomes. As models generally focus on first-line treatment without consideration of downstream treatments, there is a profound source of structural uncertainty implying that the cost-effectiveness of treatments across the mCRC pathway remains uncertain.




Similar content being viewed by others
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53.
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–9.
Australian Institue of Health and Welfare. Cancer Data in Australia. 2018. https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/acim-books. Accessed 28 Oct 2019.
American Cancer Society. Cancer Facts & Figures 2019. 2019. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html. Accessed 28 Oct 2019.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–47.
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–7.
Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18(1):136–47.
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–14.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–64.
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(14):2311–9.
Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–19.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–91.
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–9.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9.
Temraz S, Mukherji D, Shamseddine A. Sequencing of treatment in metastatic colorectal cancer: where to fit the target. World J Gastroenterol. 2014;20(8):1993–2004.
Loree JM, Kopetz S. Recent developments in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol. 2017;9(8):551–64.
Leung HW, Chan AL, Leung MS, Lu CL. Systematic review and quality assessment of cost-effectiveness analysis of pharmaceutical therapies for advanced colorectal cancer. Ann Pharmacother. 2013;47(4):506–18.
Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, von der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50(1):40–9.
Haji Ali Afzali H, Bojke L, Karnon J. Model structuring for economic evaluations of new health technologies. PharmacoEconomics. 2018;36(11):1309–19.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
International Monetary Fund. Exchange Rate Archives by Month. 2019. https://www-imf-org.ezp.lib.unimelb.edu.au/external/np/fin/data/param_rms_mth.aspx. Accessed 1 Nov 2019.
Organisation for Economic Co-operation and Development. Inflation (CPI). 2019. https://data.oecd.org/price/inflation-cpi.htm. Accessed 1 Nov 2019.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50.
Hillner BE, Schrag D, Sargent DJ, Fuchs CS, Goldberg RM. Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer. 2005;104(9):1871–84.
Tappenden P, Jones R, Paisley S, Carroll C. The cost-effectiveness of bevacizumab in the first-line treatment of metastatic colorectal cancer in England and Wales. Eur J Cancer. 2007;43(17):2487–94.
Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess. 2007;11(12):1–128, iii–iv.
Obradovic M, Mrhar A, Kos M. Cost-effectiveness of UGT1A1 genotyping in second-line, high-dose, once every 3 weeks irinotecan monotherapy treatment of colorectal cancer. Pharmacogenomics. 2008;9(5):539–49.
Gold HT, Hall MJ, Blinder V, Schackman BR. Cost effectiveness of pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 before irinotecan administration for metastatic colorectal cancer. Cancer. 2009;115(17):3858–67.
Tumeh JW, Shenoy PJ, Moore SG, Kauh J, Flowers C. A Markov model assessing the effectiveness and cost-effectiveness of FOLFOX compared with FOLFIRI for the initial treatment of metastatic colorectal cancer. Am J Clin Oncol. 2009;32(1):49–55.
Wong YN, Meropol NJ, Speier W, Sargent D, Goldberg RM, Beck JR. Cost implications of new treatments for advanced colorectal cancer. Cancer. 2009;115(10):2081–91.
KRAS testing for anti-EGFR therapy in advanced colorectal cancer: an evidence-based and economic analysis. Ont Health Technol Assess Ser. 2010;10(25):1-49.
Pichereau S, Le Louarn A, Lecomte T, Blasco H, Le Guellec C, Bourgoin H. Cost-effectiveness of UGT1A1*28 genotyping in preventing severe neutropenia following FOLFIRI therapy in colorectal cancer. J Pharm Pharm Sci. 2010;13(4):615–25.
Shiroiwa T, Motoo Y, Tsutani K. Cost-effectiveness analysis of KRAS testing and cetuximab as last-line therapy for colorectal cancer. Mol Diagn Ther. 2010;14(6):375–84.
Asseburg C, Frank M, Kohne CH, Hartmann JT, Griebsch I, Mohr A, et al. Cost-effectiveness of targeted therapy with cetuximab in patients with K-ras wild-type colorectal cancer presenting with initially unresectable metastases limited to the liver in a German setting. Clin Ther. 2011;33(4):482–97.
Blank PR, Moch H, Szucs TD, Schwenkglenks M. KRAS and BRAF mutation analysis in metastatic colorectal cancer: a cost-effectiveness analysis from a Swiss perspective. Clin Cancer Res. 2011;17(19):6338–46.
Behl AS, Goddard KA, Flottemesch TJ, Veenstra D, Meenan RT, Lin JS, et al. Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer. J Nati Cancer Inst. 2012;104(23):1785–95.
Lee EK, Revil C, Ngoh CA, Lister J, Kwon JM, Park MH, et al. Clinical and cost effectiveness of bevacizumab + FOLFIRI combination versus FOLFIRI alone as first-line treatment of metastatic colorectal cancer in South Korea. Clin Ther. 2012;34(6):1408–19.
Vijayaraghavan A, Efrusy MB, Goke B, Kirchner T, Santas CC, Goldberg RM. Cost-effectiveness of KRAS testing in metastatic colorectal cancer patients in the United States and Germany. Int J Cancer. 2012;131(2):438–45.
Hoyle M, Crathorne L, Peters J, Jones-Hughes T, Cooper C, Napier M, et al. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model. Health Technol Assess. 2013;17(14):1–237.
Hoyle M, Peters J, Crathorne L, Jones-Hughes T, Cooper C, Napier M, et al. Cost-effectiveness of cetuximab, cetuximab plus irinotecan, and panitumumab for third and further lines of treatment for KRAS wild-type patients with metastatic colorectal cancer. Value Health. 2013;16(2):288–96.
Lawrence D, Maschio M, Leahy KJ, Yunger S, Easaw JC, Weinstein MC. Economic analysis of bevacizumab, cetuximab, and panitumumab with fluoropyrimidine-based chemotherapy in the first-line treatment of KRAS wild-type metastatic colorectal cancer (mCRC). J Med Econ. 2013;16(12):1387–98.
Nebuloni DR, Mak MP, Souza FH, Saragiotto DF, Julio T, D. E. Castro G J, et al. Modified FLOX as first-line chemotherapy for metastatic colorectal cancer patients in the public health system in Brazil: effectiveness and cost-utility analysis. Mol Clin Oncol. 2013;1(1):175–9.
Ewara EM, Zaric GS, Welch S, Sarma S. Cost-effectiveness of first-line treatments for patients with KRAS wild-type metastatic colorectal cancer. Curr Oncol. 2014;21(4):e541–50.
Goldstein DA, Chen Q, Ayer T, Howard DH, Lipscomb J, Harvey RD, et al. Cost effectiveness analysis of pharmacokinetically-guided 5-fluorouracil in FOLFOX chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer. 2014;13(4):219–25.
Graham CN, Hechmati G, Hjelmgren J, de Liege F, Lanier J, Knox H, et al. Cost-effectiveness analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2014;50(16):2791–801.
Westwood M, van Asselt T, Ramaekers B, Whiting P, Joore M, Armstrong N, et al. KRAS mutation testing of tumours in adults with metastatic colorectal cancer: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014;18(62):1–132.
Freeman K, Connock M, Cummins E, Gurung T, Taylor-Phillips S, Court R, et al. Fluorouracil plasma monitoring: systematic review and economic evaluation of the My5-FU assay for guiding dose adjustment in patients receiving fluorouracil chemotherapy by continuous infusion. Health Technol Assess. 2015;19(91):1–321, v–vi.
Goldstein DA, Chen Q, Ayer T, Howard DH, Lipscomb J, El-Rayes BF, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33(10):1112–8.
Wen F, Yang Y, Zhang P, Zhang J, Zhou J, Tang R, et al. Cost-effectiveness of RAS screening before monoclonal antibodies therapy in metastatic colorectal cancer based on FIRE3 Study. Cancer Biol Ther. 2015;16(11):1577–84.
Butzke B, Oduncu FS, Severin F, Pfeufer A, Heinemann V, Giessen-Jung C, et al. The cost-effectiveness of UGT1A1 genotyping before colorectal cancer treatment with irinotecan from the perspective of the German statutory health insurance. Acta Oncol. 2016;55(3):318–28.
Graham CN, Maglinte GA, Schwartzberg LS, Price TJ, Knox HN, Hechmati G, et al. Economic analysis of panitumumab compared with cetuximab in patients with wild-type KRAS metastatic colorectal cancer that progressed after standard chemotherapy. Clin Ther. 2016;38(6):1376–91.
Riesco-Martinez MC, Berry SR, Ko YJ, Mittmann N, Giotis A, Lien K, et al. Cost-effectiveness analysis of different sequences of the use of epidermal growth factor receptor inhibitors for wild-type KRAS unresectable metastatic colorectal cancer. J Oncol Pract. 2016;12(6):e710–23.
Zhou J, Zhao R, Wen F, Zhang P, Tang R, Chen H, et al. Economic evaluation study (CHEER-compliant): Cost-effectiveness analysis of RAS screening for treatment of metastatic colorectal cancer based on the CALGB 80405 trial. Medicine (Baltimore). 2016;95(27):e3762.
Carvalho AC, Leal F, Sasse AD. Cost-effectiveness of cetuximab and panitumumab for chemotherapy-refractory metastatic colorectal cancer. PLoS One. 2017;12(4):e0175409.
Franken MD, van Rooijen EM, May AM, Koffijberg H, van Tinteren H, Mol L, et al. Cost-effectiveness of capecitabine and bevacizumab maintenance treatment after first-line induction treatment in metastatic colorectal cancer. Eur J Cancer. 2017;75:204–12.
Goldstein DA, Chen Q, Ayer T, Chan KKW, Virik K, Hammerman A, et al. Bevacizumab for metastatic colorectal cancer: a global cost-effectiveness analysis. Oncologist. 2017;22(6):694–9.
Huxley N, Crathorne L, Varley-Campbell J, Tikhonova I, Snowsill T, Briscoe S, et al. The clinical effectiveness and cost-effectiveness of cetuximab (review of technology appraisal no. 176) and panitumumab (partial review of technology appraisal no. 240) for previously untreated metastatic colorectal cancer: a systematic review and economic evaluation. Health Technol Assess. 2017;21(38):1–294.
Parikh RC, Du XL, Robert MO, Lairson DR. Cost-effectiveness of treatment sequences of chemotherapies and targeted biologics for elderly metastatic colorectal cancer patients. J Manag Care Spec Pharm. 2017;23(1):64–73.
Rivera F, Valladares M, Gea S, Lopez-Martinez N. Cost-effectiveness analysis in the Spanish setting of the PEAK trial of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. J Med Econ. 2017;20(6):574–84.
Saito S, Kameyama H, Muneoka Y, Okuda S, Wakai T, Akazawa K. Cost-effectiveness analysis of the use of comprehensive molecular profiling before initiating monoclonal antibody therapy against metastatic colorectal cancer in Japan. J Cancer Policy. 2017;12:61–6.
Toumazis I, Kurt M, Toumazi A, Karacosta LG, Kwon C. Comparative effectiveness of up to three lines of chemotherapy treatment plans for metastatic colorectal cancer. MDM Policy Pract. 2017;2(2):2381468317729650.
Ungari AQ, Pereira LRL, Nunes AA, Peria FM. Cost-effectiveness analysis of XELOX versus XELOX plus bevacizumab for metastatic colorectal cancer in a public hospital school. BMC Cancer. 2017;17(1):691.
Wu B, Yao Y, Zhang K, Ma X. RAS testing and cetuximab treatment for metastatic colorectal cancer: a cost-effectiveness analysis in a setting with limited health resources. Oncotarget. 2017;8(41):71164–72.
Bolaños-Díaz R, Sanabria-Montañez C, Farfán-Tello C, Calderón-Cahua M. Cost-effectiveness of Cetuximab as a treatment strategy for metastatic colon cancer in Peru: chemotherapy/Cetuximab versus chemotherapy alone. J Pharm Health Serv Res. 2018;9(4):319–26.
Bullement A, Underhill S, Fougeray R, Hatswell AJ. Cost-effectiveness of trifluridine/tipiracil for previously treated metastatic colorectal cancer in England and Wales. Clin Colorectal Cancer. 2018;17(1):e143–51.
Cho SK, Hay JW, Barzi A. Cost-effectiveness analysis of regorafenib and TAS-102 in refractory metastatic colorectal cancer in the United States. Clin Colorectal Cancer. 2018;17(4):e751–61.
Degeling K, Franken MD, May AM, van Oijen MGH, Koopman M, Punt CJA, et al. Matching the model with the evidence: comparing discrete event simulation and state-transition modeling for time-to-event predictions in a cost-effectiveness analysis of treatment in metastatic colorectal cancer patients. Cancer Epidemiol. 2018;57:60–7.
Graham CN, Christodoulopoulou A, Knox HN, Sabatelli L, Hechmati G, Garawin T, et al. A within-trial cost-effectiveness analysis of panitumumab compared with bevacizumab in the first-line treatment of patients with wild-type RAS metastatic colorectal cancer in the US. J Med Econ. 2018;21(11):1075–83.
Harty G, Jarrett J, Jofre-Bonet M. Consequences of biomarker analysis on the cost-effectiveness of cetuximab in combination with FOLFIRI as a first-line treatment of metastatic colorectal cancer: personalised medicine at work. Appl Health Econ Health Policy. 2018;16(4):515–25.
Shankaran V, Ortendahl JD, Purdum AG, Bolinder B, Anene AM, Sun GH, et al. Cost-effectiveness of cetuximab as first-line treatment for metastatic colorectal cancer in the United States. Am J Clin Oncol. 2018;41(1):65–72.
Tikhonova IA, Huxley N, Snowsill T, Crathorne L, Varley-Campbell J, Napier M, et al. Economic analysis of first-line treatment with cetuximab or panitumumab for RAS wild-type metastatic colorectal cancer in England. PharmacoEconomics. 2018;36(7):837–51.
Uyl-de Groot CA, van Rooijen EM, Punt CJA, Pescott CP. Real-world cost-effectiveness of cetuximab in the third-line treatment of metastatic colorectal cancer based on patient chart review in the Netherlands. Health Econ Rev. 2018;8(1):13.
Xu Y, Hay JW, Barzi A. Impact of drug substitution on cost of care: an example of economic analysis of cetuximab versus panitumumab. Cost Eff Resour Alloc. 2018;16:30.
Chu JN, Choi J, Ostvar S, Torchia JA, Reynolds KL, Tramontano A, et al. Cost-effectiveness of immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Cancer. 2019;125(2):278–89.
Gourzoulidis G, Maniadakis N, Petrakis D, Souglakos J, Pentheroudakis G, Kourlaba G. Economic evaluation of trifluridine and tipiracil hydrochloride in the treatment of metastatic colorectal cancer in Greece. J Comp Eff Res. 2019;8(3):133–42.
Sherman SK, Lange JJ, Dahdaleh FS, Rajeev R, Gamblin TC, Polite BN, et al. Cost-effectiveness of maintenance capecitabine and bevacizumab for metastatic colorectal cancer. JAMA Oncol. 2019;5(2):236–42.
Zhang PF, Wen F, Zhou J, Huang JX, Zhou KX, Wu QJ, et al. Cost-effectiveness analysis of capecitabine plus bevacizumab versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer from Chinese societal perspective. Clin Transl Oncol. 2019. https://doi.org/10.1007/s12094-019-02114-x.
National Institute for Health and Clinical Excellence. Capecitabine and tegafur uracil for metastatic colorectal cancer. NICE technology appraisal TA61. 2012. http://guidance.nice.org.uk/TA61. Accessed 15 Nov 2019.
Whyte S, Pandor A, Stevenson M. Bevacizumab for metastatic colorectal cancer: a NICE single technology appraisal. PharmacoEconomics. 2012;30(12):1119–32.
Woods B, Sideris E, Palmer S, Latimer N, Soares M. NICE DSU Technical Support Document 19. Partitioned survival analysis for decision modelling in health care: a critical review. 2017. http://www.nicedsu.org.uk. Accessed 15 Nov 2019.
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–57 (discussion 57–8).
Burbach JP, Kurk SA, Coebergh van den Braak RR, Dik VK, May AM, Meijer GA, et al. Prospective Dutch colorectal cancer cohort: an infrastructure for long-term observational, prognostic, predictive and (randomized) intervention research. Acta Oncol. 2016;55(11):1273–80.
Field K, Wong HL, Shapiro J, Kosmider S, Tie J, Bae S, et al. Developing a national database for metastatic colorectal cancer management: perspectives and challenges. Intern Med J. 2013;43(11):1224–31.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Value Health. 2012;15(6):843–50.
Vemer P, Corro Ramos I, van Voorn GAK, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. PharmacoEconomics. 2016;34(4):349–61.
Degeling K, IJzerman MJ, Koopman M, Koffijberg H. Accounting for parameter uncertainty in the definition of parametric distributions used to describe individual patient variation in health economic models. BMC Med Res Methodol. 2017;17(1):170.
Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Van Krieken JH, Rouleau E, Ligtenberg MJ, Normanno N, Patterson SD, Jung A. RAS testing in metastatic colorectal cancer: advances in Europe. Virchows Arch. 2016;468(4):383–96.
Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–6.
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–15.
Deenen MJ, Meulendijks D, Cats A, Sechterberger MK, Severens JL, Boot H, et al. Upfront genotyping of DPYD*2A to individualize fluoropyrimidine therapy: a safety and cost analysis. J Clin Oncol. 2016;34(3):227–34.
Henricks LM, Lunenburg C, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E, et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 2018;19(11):1459–67.
Henricks LM, Lunenburg C, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E, et al. A cost analysis of upfront DPYD genotype-guided dose individualisation in fluoropyrimidine-based anticancer therapy. Eur J Cancer. 2019;107:60–7.
Bentley TG, Broder MS, Das L, Ortendahl J, Su Y, Wagner S. Targeted therapies for metastatic colorectal cancer (mCRC): A systematic review of cost-effectiveness (CE). J Clin Oncol. 2012;30(Suppl 4):583.
Bullement A, Cranmer HL, Shields GE. A review of recent decision-analytic models used to evaluate the economic value of cancer treatments. Appl Health Econ Health Policy. 2019. https://doi.org/10.1007/s40258-019-00513-3.
Acknowledgements
The authors would like to thank Lindy Cochrane, Liaison Librarian, Nursing, Health Sciences, Population and Global Health at the University of Melbourne, for critically reviewing and advising on the selection of databases and search strategy.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The search strategy was prepared by KD, MV and MIJ, and critically reviewed and approved by all other authors. The selection process and data extraction were performed by KD and MV. All authors contributed to the interpretation and discussion of the results. The first draft of the manuscript was prepared by KD, and critically reviewed by all other authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
No funding was received for performing this study.
Conflicts of interest
Authors KD, HK, MK and MIJ acknowledge authorship on several publications that were included in the analysis. MK is advisor to The Netherlands Organisation for Health Research and Development, a member of the scientific board of the Dutch Cancer Society (KWF), chair of the Dutch Colorectal Cancer Group (DCCG), principal investigator (PI) for PLCRC (Dutch national observational cohort study), involved in several clinical trials in CRC as PI or co-investigator and declares unrestricted institutional grants from Amgen, Bayer, BMS, Merck-Serono, Nordic Farma, Roche, Servier, Sirtex and Sanofi-Aventis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Degeling, K., Vu, M., Koffijberg, H. et al. Health Economic Models for Metastatic Colorectal Cancer: A Methodological Review. PharmacoEconomics 38, 683–713 (2020). https://doi.org/10.1007/s40273-020-00908-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-020-00908-4