Abstract
Background and Objective
Substantial adjuvant chemotherapy (AC) overtreatment for stage II colorectal cancer results in a health and financial burden. Circulating tumour DNA (ctDNA) can improve patient selection for AC by detecting micro-metastatic disease. We estimated the health economic potential of ctDNA-guided AC for stage II colorectal cancer.
Methods
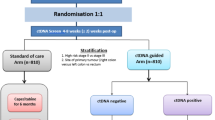
A cost-utility analysis was performed to compare ctDNA-guided AC to standard of care, where 22.6% of standard of care patients and all ctDNA-positive patients (8.7% of tested patients) received AC and all ctDNA-negative patients (91.3%) did not. A third preference-sensitive ctDNA strategy was included where 6.8% of ctDNA-negative patients would receive AC. A state-transition model was populated using data from a prospective cohort study and clinical registries. Health and economic outcomes were discounted at 5% over a lifetime horizon from a 2019 Australian payer perspective. Extensive scenario and probabilistic analyses quantified model uncertainty.
Results
Compared to standard of care, the ctDNA and preference-sensitive ctDNA strategies increased quality-adjusted life-years by 0.20 (95% confidence interval − 0.40 to 0.81) and 0.19 (− 0.40 to 0.78), and resulted in incremental costs of AUD − 4055 (− 16,853 to 8472) and AUD − 2284 (− 14,685 to 10,116), respectively. Circulating tumour DNA remained cost effective at a willingness to pay of AUD 20,000 per quality-adjusted life-year gained throughout most scenario analyses in which the proportion of ctDNA-positive patients cured by AC and compliance to a ctDNA-negative test results were decreased.
Conclusions
Circulating tumour-guided AC is a potentially cost-effective strategy towards reducing overtreatment in stage II colorectal cancer. Results from ongoing randomised clinical studies will be important to reduce uncertainty in the estimates.



Similar content being viewed by others
References
Benson AB, Venook AP, Al-Hawardy MM, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(7):874–901.
Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv22-40.
Benson AB, Venook AP, Al-Hawary MM, et al. Colon Cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329–59. https://doi.org/10.6004/jnccn.2021.0012.
Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92.
Schrag D, Rifas-Shiman S, Saltz L, et al. Adjuvant chemotherapy use for medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20(19):3999–4005.
Figueredo A, Charette ML, Maroun J, et al. Adjuvant therapy for stage II colon. Cochrane Database Syst Rev. 2008;3:1465–858.
Kannarkatt J, Joseph J, Kurniali PC, Al-Janadi A, Hrinczenko B. Adjuvant chemotherapy for stage II colon cancer: a clinical dilemma. J Oncol Pract. 2017;13(4):233–41.
André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–51.
Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–9.
International Multicentre Pooled Analysis of Colon Cancer Trials Investigators. Efficacy of adjuvant fluorouracil and folinic acid in B2 and Colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17:1356–63.
André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–16.
Wan JCM, Massie C, Garcioa-Corbacho J, et al. Liquid biopsies come of age: towards implentation of circulating tumor DNA. Nat Rev Cancer. 2017;17:223–8.
Bettegowd C, Sausen M, Leary R, et al. Detection of circulating tumour DNA in early- and late-stage human malignanies. Sci Transl Med. 2014;6(224):224ra24.
Tie J, Cohen JD, Wang Y, Li L, Christie M, Simons K, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut. 2019;68(4):663–71.
Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol. 2019;5(12):1710–7.
Tarazon N, Gimeno-Valiente F, Gambardella V, et al. Targeted next-generation sequencing of circulating tumour DNA for tracking minimal residual disesae in localised colon cancer. Ann Oncol. 2019;30(11):1804–12.
Reinert T, Heriksen TV, Christensen E, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124–31.
Khakoo S, Carter PD, Brown G, et al. MRI tumour regression grade and circulating tumour DNA as complementary tools to assess response and guide therapy adapation in rectal cancer. Clin Cancer Res. 2020;26(1):183–92.
Boer RH, Baker C, Speakman D, et al. The impact of a genomic assay (Oncotype Dx) on adjuvant treatment recommendations in early breast cancer. Med J Aust. 2013;199(3):205–8.
Sakata S, Cronk M. The financial burden of using Oncotype Dx for patients with lymph node negative and estrogen receptor-positive breast cancer in Australia. Asia Pac J Clin Oncol. 2014;10(1):94–5.
Medical Services Advisory Committee. Application no. 1342.5: gene expression profiling of 21 genes in breast cancer to quanity the risk of disease recurrence and predict adjuvant chemotherapy benefit. 2019. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/F110B75361D91B5DCA2583CF00166434/$File/1342.5%20-%20Final%20PSD.pdf. Accessed 16 Dec 2020.
Medical Services Advisory Committee. Application no. 1376.1: 70 gene signature (Mammaprint) for use in breast cancer to quantify the risk of disease recurren and predict adjuvant chemotherapy benefit. 2018. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/C7E58C0AE3D9CD7ECA2580E9007F867D/$File/1376.1%20-%20Final%20PSD.pdf. Accessed 16 Dec 2020.
Biogrid Australia. 2020. www.biogrid.com.au. Accessed 25 May 2021.
Field K, Wong HL, Shapiro J, et al. Developing a national database for metastatic colorectal cancer management: perspectives and challenges. Intern Med J. 2013;43(11):1224–31.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28(2):264–71.
Bockelman C, Engelmann B, Kaprio T, et al. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54(1):5–16.
Tsikitis V, Larson DW, Huebner M, et al. Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer. 2014;14:336.
Australian Bureau of Statistics. Life tables, states, territories and Australia, 2016–2018. Canberra, ABS. https://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/97E435FA3B82A89DCA2570A6000573D3?opendocument. Accessed 15 Sep 2020.
Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra D. Preference values associated with stage III colon cancer and adjuvant chemotherpay. Qual Life Res. 2010;19:391–400.
Carlson J, Roth J. The impact of Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141(1):13–22.
Wuerstlein R, Kates R, Gluz O, et al. Strong impact of MammaPrint and BluePrint on treatment decision in luminal early breast cancer: results of the WSG-PRIMe study. Breast Cancer Res Treat. 2019;175(2):289–99.
Kerr RS, Love S, Segelov E, Johnston E, Falcon B, Hewett P. Adjuvant capecitabine plus bevacizumab versus capecitabine alone in patients with colorectal cancer (QUASAR 2): an open-label, randomised phase 3 trial. Lancet. 2016;17(11):1543–57.
Australian Institute of Health and Welfare. Health expenditure Australia 2018–2019. Health and welfare expenditure series no. 66. Cat no. HWE 80. 2019. Canberra: Australian Institute of Health and Welfare; 2020.
Pharmaceutical Benefit Schedule. PBS publication archives[internet]. Canberra: Pharmaceutical Benefit Schedule. [updated: 2021 April 1. Cited: 2021 May 05]. Available from: https://www.pbs.gov.au/info/publication/schedule/archive.
Medicare Benefit Schedule. MBS Online Downloads [internet]. Canberra: Medicare Benefit Schedule [updated: 2021 March 09. Cited 2021 May 05]. Available from: http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/downloads.
National Hospital Cost Data Collection. AR-DRG cost weight tables V8.0x, round 21 (financial year 2016–17). Canberra: Independent Hospital Pricing Authority; 2019.
Ananda S, Kosmider S, Tran B, Field K, et al. The rapidly escalating cost of treating colorectal cancer in Australia. Asia Pac J Clin Oncol. 2016;12:33–40.
Latimer NR. Survival analysis for economic evaluations alongside clinical trials: extrapolation with patient-level data: inconsistencies, limitations and a practical guide. Med Decis Making. 2013;33(60):743–5.
Austin P, Stuart E. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using propensity score to estimate casual treatment in observational studies. Stat Med. 2015;34(28):3661–79.
Buuren SV. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–42.
Australian Government Department of Health. Technical guidelines for preparing assessment reports for the Medical Services Advisory Committee—service type: investigative (version 3.0). Canberra: Australian Government Department of Health; 2017.
Caro JJ, Briggs AH, Siebert U, et al. Modelling good research practices: overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health. 2012;15(5):796–803.
Degeling K, Ijzerman M, Koopman M, et al. Accounting for parameter uncertainty in the definition of parametric distributions used to describe inidividual patient variation in health economiic models. BMC Med Red Methodol. 2017;17:170.
Australian Bureau of Statistics. Average weekly earnings, Australia [internet]. Canberra: Australian Bureau of Statistics. [updated: 2020 Feb 20. Cited: 2021 May 05]. Available from: https://www.abs.gov.au/statistics/labour/earnings-and-work-hours/average-weekly-earnings-australia/nov-2019.
Buisman LR, Rutten-van Mölken M, Postmus D, Luime JJ, Uyl-de Groot CA, Redekop WK. The early bird catches the worm: early cost-effectiveness analysis of new medical tests. Int J Technol Assess Health Care. 2016;32(1):1–8.
Ijzerman MJ, Steuten LM. Early assessment of medical technologies to inform product development and market access. Appl Health Econ Health Policy. 2011;9(5):331–47.
Jongeneel G, Greuter MJE, van Erning FN, et al. Model-based effectiveness and cost-efectivness of risk-based selection strategies for adjuvant chemotherapy in Dutch stage II colon cancer patients. Therap Adv Gastroenterol. 2021;14:1756284821995715.
Jongeneel G, Greuter MJE, van Erning FN, et al. Modelling Personalied Adjuvant TreaTment in EaRly stage coloN cancer (PATTERN). Eur J Health Econ. 2020;21:1059–73.
Kopetz S, Tabernero J, Rosenberg R, et al. Genomic classifier ColoPrint predicts recurrence in stage II colorectal cancer patients more accurately than clinical factors. Oncologist. 2015;20(2):127–33.
Alberts SR, Yu TM, Behrens RJ, et al. Comparative economics of a 12-gene assay for predicinting risk of recurrence in stage II colon cancer. Pharmacoeconomics. 2014;32:1231–43.
Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–7.
Kocina E, Haan S, Rauh S, et al. Prognostic and predictive molecular biomarkers for colroectal cancer: updates and challenges. Cancers. 2020;12(2):319.
Bratman SV, Newman AM, Alizadeh AA, et al. Potential clinical utility of ultrasensitive circulating tumour DNA detection with CAPP-Seq. Expert Rev Mol Diagn. 2015;15:715–9.
Guardant Health Inc. The Guardant260 Assay receives expedited access pathway designation for breakthrough devices from FDA. Redwood City: Guardant Health. [updated: 2018 February 15. Cited: 2020 Oct 19]. Available from: https://investors.guardanthealth.com/press-releases/press-releases/2018/The-Guardant360-Assay-Receives-Expedited-Access-Pathway-Designation-for-Breakthrough-Devices-from-FDA/default.aspx.
Acknowledgements
The authors acknowledge BioGrid Australia for providing data access and support to the ACCORD and TRACC databases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the conduct of this study or the preparation of this article. Roche Product Pty Limited (Australia) provided financial assistance for the development, installation and maintenance of the TRACC database, data from which were used in this analysis.
Conflicts of interest/Competing interests
Yat Hang To, Koen Degeling, Suzanne Kosmider, Rachel Wong, Margaret Lee, Catherine Dunn, Grace Gard, Azim Jalali, Vanessa Wong, Maarten IJzerman, Peter Gibbs and Jeanne Tie have no conflicts of interests that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Availability of data and material
The data generated during and/or analysed during the current study are available from the corresponding author, upon reasonable request.
Code availability
The custom codes generated for this study are available from the corresponding author, upon reasonable request.
Author contributions
YHT, KD and JT contributed to the study conception and design. Material preparation, data collection and analysis were performed by YHT under supervision of KD. Further supervision was provided by JT. All authors contributed to the interpretation and discussion of the results. The initial draft of the manuscript was written by YHT in collaboration with KD, and critically reviewed by all other authors. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
To, Y.H., Degeling, K., Kosmider, S. et al. Circulating Tumour DNA as a Potential Cost-Effective Biomarker to Reduce Adjuvant Chemotherapy Overtreatment in Stage II Colorectal Cancer. PharmacoEconomics 39, 953–964 (2021). https://doi.org/10.1007/s40273-021-01047-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-021-01047-0