Abstract
Background
Patients with highly active relapsing-remitting multiple sclerosis inadequately responding to first-line therapies (interferon-based therapies, glatiramer acetate, dimethyl fumarate, and teriflunomide, known collectively as “BRACETD”) often switch to natalizumab or fingolimod.
Objective
The aim was to estimate the comparative effectiveness of switching to natalizumab or fingolimod or within BRACETD using real-world data and to evaluate the cost-effectiveness of switching to natalizumab versus fingolimod using a United Kingdom (UK) third-party payer perspective.
Methods
Real-world data were obtained from MSBase for patients relapsing on BRACETD in the year before switching to natalizumab or fingolimod or within BRACETD. Three-way-multinomial-propensity-score–matched cohorts were identified, and comparisons between treatment groups were conducted for annualised relapse rate (ARR) and 6-month–confirmed disability worsening (CDW6M) and improvement (CDI6M). Results were applied in a cost-effectiveness model over a lifetime horizon using a published Markov structure with health states based on the Expanded Disability Status Scale. Other model parameters were obtained from the UK MS Survey 2015, published literature, and publicly available UK sources.
Results
The MSBase analysis found a significant reduction in ARR (rate ratio [RR] = 0.64; 95% confidence interval [CI] 0.57–0.72; p < 0.001) and an increase in CDI6M (hazard ratio [HR] = 1.67; 95% CI 1.30–2.15; p < 0.001) for switching to natalizumab compared with BRACETD. For switching to fingolimod, the reduction in ARR (RR = 0.91; 95% CI 0.81–1.03; p = 0.133) and increase in CDI6M (HR = 1.30; 95% CI 0.99–1.72; p = 0.058) compared with BRACETD were not significant. Switching to natalizumab was associated with a significant reduction in ARR (RR = 0.70; 95% CI 0.62–0.79; p < 0.001) and an increase in CDI6M (HR = 1.28; 95% CI 1.01–1.62; p = 0.040) compared to switching to fingolimod. No evidence of difference in CDW6M was found between treatment groups. Natalizumab dominated (higher quality-adjusted life-years [QALYs] and lower costs) fingolimod in the base-case cost-effectiveness analysis (0.453 higher QALYs and £20,843 lower costs per patient). Results were consistent across sensitivity analyses.
Conclusions
This novel real-world analysis suggests a clinical benefit for therapy escalation to natalizumab versus fingolimod based on comparative effectiveness results, translating to higher QALYs and lower costs for UK patients inadequately responding to BRACETD.
Similar content being viewed by others
The increasing availability of real-world evidence in multiple sclerosis (MS) allows for a novel collaborative effort to design and conduct a real-world registry analysis of highly active MS treatment escalation alternatives in alignment with established cost-effectiveness modelling approaches for MS. |
Results from the MSBase registry suggest that treatment escalation to natalizumab is more effective on relapse- and disability improvement-based outcomes compared to switching between BRACETD treatments or escalating to fingolimod. |
Results from our cost-effectiveness analysis also indicate that switching to natalizumab improves lifetime clinical and economic outcomes compared with switching to fingolimod for patients with highly active relapsing-remitting MS with inadequate response to first-line therapies from a United Kingdom payer perspective. |
1 Introduction
Cost-effectiveness analyses are the cornerstone of health technology appraisals (HTAs) used by many payers globally to inform drug pricing or reimbursement decisions. Traditionally, such analyses have focused on newly approved drugs and relied primarily on clinical trial evidence. However, with the emergence of longitudinal disease registries and the expansion of treatment alternatives in conditions such as multiple sclerosis (MS), payers and HTA agencies are increasingly requesting real-world evidence when re-evaluating drugs that are already reimbursed.
Large disease registries, like the international MSBase registry [1], frequently provide independent analysis for clinical comparisons. This paper describes a novel collaborative effort to design a real-world registry analysis of MS treatments in alignment with established health economic modelling requirements in MS. Acknowledging that the methodologies and terminologies for real-world clinical outcome analysis in MS and for health economic (or pharmacoeconomic) analysis differ meaningfully, this paper represents a unique effort in the MS literature to join these approaches in pursuit of improved understanding of the benefits and value of treatment alternatives in MS.
Disease-modifying therapies (DMTs) for relapsing-remitting MS (RRMS), the most common MS subtype, reduce the frequency of relapses and the accumulation of disability that contribute to the burden of MS for patients and caregivers [2,3,4,5]. The prevailing treatment strategy in RRMS involves escalation of DMTs, where patients with inadequate response to first-line treatment options (interferon-based therapies [Betaferon®/Betaseron (US)®, Rebif®, Avonex®, Extavia®], glatiramer acetate [Copaxone®], teriflunomide [Aubagio®], and dimethyl fumarate [Tecfidera®], known collectively as “BRACETD”) switch to more effective therapies, such as natalizumab (Tysabri®) or fingolimod (Gilenya®), among others [5]. While these escalation DMTs are more efficacious, they carry increased risks of rare but serious adverse events (AEs) (e.g. progressive multifocal leukoencephalopathy [PML], secondary autoimmunity, cardiac arrhythmias, and breast cancer) [6]. Since relapses remain the most widespread measure of disease activity, at least one relapse in the year prior to switching therapy was used to define a patient with inadequate response to MS treatment. This definition mirrors the current approved European Medicines Agency regulatory indication for both natalizumab and fingolimod; however, it is recognised that this definition continues to evolve in the scientific community.
Real-world evidence has an established role in characterising the natural history and burden of RRMS in economic evaluations of DMTs [7,8,9,10]. Real-world data (RWD) from disease registries such as MSBase [1] are increasingly used to study the comparative effectiveness of DMTs for RRMS, especially escalation alternatives such as natalizumab and fingolimod [11, 12]. The objective of this study was to design and conduct a comparative effectiveness analysis using RWD from MSBase for use in a pharmacoeconomic model to estimate the cost-effectiveness from a United Kingdom (UK) third-party payer perspective of switching to natalizumab compared with switching to fingolimod in patients with highly active RRMS (HA-RRMS) with inadequate response to BRACETD.
2 Methods
2.1 Study Population
The population for the comparative effectiveness and cost-effectiveness analyses comprised adults with HA-RRMS with inadequate response (defined as ≥ 1 relapse in the year before switching) after ≥ 1 year on a first-line DMT (BRACETD) who switched to natalizumab, fingolimod, or another BRACETD. Patients switching to another BRACETD were included as a common reference group in the comparative effectiveness analysis to facilitate extrapolation and post-discontinuation scenarios for natalizumab and fingolimod in the cost-effectiveness analysis.
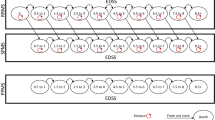
To identify this population in the MSBase registry, longitudinal data from 42,189 patients across 139 MS centres in 39 countries were extracted from the registry database on 13 June 2016. These centres are requested to perform at least a yearly clinical assessment of their patients, although this was not used as an exclusion criterion for the current study. Patients aged ≥ 18 years with RRMS were included in the study population if they met the switch criteria (defined in the paragraph above), had completed ≥ 1 year of uninterrupted treatment with BRACETD before switching, and had ≥ 1 relapse in the year before switching while on BRACETD. The analysis excluded patients with non-BRACETD MS treatments prior to switching. A maximum 6-month treatment gap was allowed at the time of switching, and a minimum 3-month treatment persistence after switching was required. Complete data for all predefined matching variables [baseline patient age, sex, residential country, index year, and other disease and history variables (Table 1)] were included in the three-way-multinomial-propensity-score matching algorithm [13]. The matching variables were chosen based on availability in the MSBase registry and known correlation with treatment outcome based on published literature and previous MSBase research [11, 12]. Baseline, or time of switch, was defined as the date at which natalizumab, fingolimod, or another BRACETD DMT was initiated. Propensity scores were derived for the natalizumab, fingolimod and BRACETD cohorts using a logistic regression with treatment group as the dependent variable. The predefined matching variables were the independent model covariates. Table S1 (see the Electronic Supplementary Material) presents values for all required variables before matching. Figure 1 presents a Consolidated Standards of Reporting Trials (CONSORT)-like diagram detailing patient selection.
Patient selection flow chart for MSBase analysis. BRACETD interferon-based therapies, glatiramer acetate, dimethyl fumarate, and teriflunomide, RRMS relapsing-remitting multiple sclerosis. aRequires completion of ≥ 12 months uninterrupted treatment with a BRACETD therapy and a < 6-month gap between discontinuing the BRACETD therapy and initiating the switch therapy. bAll variables included in the propensity score matching algorithm (Table 1 in the main text) were required for inclusion in the analysis
2.2 Comparative Effectiveness Analysis
2.2.1 Outcome Measures
We captured all data included in eligible patients’ records beginning at the time of switching and ending with discontinuation of the switch therapy or conversion to secondary progressive MS (SPMS) or last Expanded Disability Status Scale (EDSS) visit recorded in MSBase. All MSBase participating centres are required to have Neurostatus certification to perform EDSS assessments. Comparative effectiveness was assessed for four outcomes: annualised relapse rate (ARR), time to first relapse, time to 6-month–confirmed disability worsening (CDW6M), and time to 6-month–confirmed disability improvement (CDI6M).
A relapse was defined as occurrence of new symptoms or exacerbation of existing symptoms persisting for at least 24 h, in the absence of concurrent illness or fever, and occurring at least 30 days after a previous relapse [14]. Relapses were confirmed by the treating physician; confirmation with EDSS scores or magnetic resonance imaging (MRI) was not required. CDW6M was defined as an increase in EDSS score of at least 1 point from baseline sustained between two follow-up visits separated in time by no less than 6 months (1.5 points if EDSS at baseline was 0; 0.5 points if the baseline EDSS ≥ 5.5). CDI6M was defined as a decrease in EDSS score of at least 1 point from a baseline EDSS equal to or above 2 sustained between two follow-up visits separated in time by no less than 6 months. EDSS scores within 30 days of a relapse were not considered for CDW6M or CDI6M. Similarly to previous studies [11], CDW6M and CDI6M were modelled as separate outcomes. Six-month confirmation of disability changes was required for the primary analysis based on regulatory preferences [15]. A 3-month confirmation definition was used in a scenario analysis.
For use in the cost-effectiveness analysis, EDSS transition probability matrices were estimated. The entries in these matrices reflect the probabilities of moving between pairs of EDSS scores during a yearlong interval. For the transition matrix analysis, confirmation of changes in EDSS were not required so that patients could contribute multiple distinct yearly intervals to the analysis.
2.2.2 Statistical Analysis
All statistical analyses were performed using Stata version 16 (RRID:SCR_012763) and R Project for Statistical Computing version 3.6.3 (RRID:SCR_001905). We compared outcomes between the three treatment groups. We used a generalised estimated equation Poisson regression model to analyse ARRs, overall and by baseline EDSS. Time to first relapse, disability worsening, and disability improvement utilised a Kaplan–Meier approach and a marginal Cox regression model that adjusted for matching but not for any other covariates. Disability worsening and improvement analyses required the inclusion of data from patients with ≥ 3 EDSS scores (baseline, change from baseline, and confirmation). Statistical tests appropriate for the clustered nature of the matched design were used. Matched triplets were censored at treatment end or last-recorded on-treatment visit. The p value for the significance threshold was set at 0.05.
In the transition matrix analysis, EDSS scores at the beginning and end of distinct yearly intervals were identified for all patients in each matched cohort. EDSS scores reported within 90 days of each time point were considered valid, and non-integer EDSS scores were rounded down in alignment with the cost-effectiveness model structure. All valid yearly intervals were used to estimate the probability of transitioning between each possible pair of EDSS scores within a year [16]. Following Markovian principles, all valid yearly intervals were given the same weight, including multiple intervals observed for the same patient and intervals occurring during different years of the follow-up period.
2.3 Cost-Effectiveness Analysis
We developed a cost-effectiveness model in alignment with UK guidelines [17] to compare predicted costs and health outcomes and to estimate the cost-effectiveness of switching to natalizumab compared with switching to fingolimod in patients with HA-RRMS with inadequate response to BRACETD. The model was programmed in Microsoft Excel (RRID:SCR_016137) for Windows with Visual Basic for Applications (Microsoft Corporation, Redmond, Washington).
2.3.1 Modelling Approach
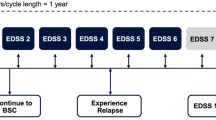
The model tracked a cohort of patients with RRMS over their remaining lifetimes as they experienced disability worsening or improvement and relapses associated with RRMS, conversion to SPMS, disability worsening and relapses associated with SPMS, and death. We used a Markov-based structure with an annual cycle length and integer EDSS-based health states, consistent with previous cost-effectiveness analyses (Fig. 2) [10, 18]. The analysis was conducted over a lifetime horizon from a UK National Health Service perspective. Scenario analyses considered shorter time horizons and a societal perspective.
Model structure diagram for cost-effectiveness analysis. BRACETD interferon-based therapies, glatiramer acetate, dimethyl fumarate, and teriflunomide, EDSS Expanded Disability Status Scale, RRMS relapsing-remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis. Note: While not shown in the figure, EDSS changes of more than one level are permitted. aDeath is reachable from all health states
The model structure included separate EDSS health states for RRMS (EDSS 0–9.0) and for SPMS (EDSS 1.0–9.0). Disability worsening was possible in both RRMS and SPMS; disability improvement was assumed possible in RRMS only. Conversion from RRMS to SPMS was assumed to be associated with a 1-point EDSS increase, consistent with modelling precedent in MS, based on the expectation that conversion to SPMS increases disability. Transitions of ≥ 1.0 EDSS score in a single cycle were allowed.
The cost-year was 2019 Great British Pound (GBP; £). All costs and health outcomes were discounted at 3.5% annually [17]. Alternative discounting rates were considered in scenario analysis.
2.3.2 Data Sources
Data were drawn from RWD sources wherever possible, with synthesised clinical trial data, the published literature, and other publicly available data sources used when necessary (Figure S1, see the Electronic Supplementary Material).
2.3.2.1 Clinical Data from MSBase
The primary RRMS clinical data used in the cost-effectiveness analysis were drawn from the results of the MSBase comparative effectiveness analysis: baseline patient age, sex, and EDSS distribution (Table S3, see the Electronic Supplementary Material); hazard ratios (HRs) for disability worsening and improvement and rate ratios (RRs) for relapses for natalizumab and fingolimod (reference = BRACETD) (Table 2 and Tables S4–S7); and treatment-specific annual EDSS transition probability matrices and ARRs for each MSBase treatment group (Tables S9–S12). The MSBase results estimated for the matched cohorts were used for the base-case cost-effectiveness analysis; estimates for the unmatched cohorts were considered in scenario analysis.
Because the MSBase cohorts had insufficient patient numbers at EDSS ≥ 7.0, we followed precedent from the MS modelling literature [10, 18] and used transition probabilities from the British Columbia Multiple Sclerosis (BCMS) database [8] and ARRs from a previous analysis [19] (Table S8) to estimate these values. The transition probability matrix and ARR extrapolation methodologies are described in Tables S9 and S10, respectively, for the matched BRACETD cohorts. The same methodologies were used for the matched natalizumab and fingolimod cohorts (Tables S11 and S12).
The MSBase analysis provided two options for modelling the effectiveness of natalizumab and fingolimod: (1) direct use of treatment-specific EDSS transition matrices and ARRs by EDSS for natalizumab and fingolimod; and (2) application of HRs for disability worsening and improvement to the BRACETD EDSS transition matrix and RRs for relapse to the ARRs for BRACETD, yielding comparative effectiveness-adjusted transition matrices and ARRs for natalizumab and fingolimod. In the base-case cost-effectiveness analysis, the model used the first option for the follow-up duration observed in MSBase before switching to the second option for long-term extrapolation. The use of each approach individually was considered in scenario analysis.
2.3.2.2 Other Clinical Data
Treatment-specific dosing regimens, including administration and monitoring requirements, for natalizumab (300 mg every 4 weeks) and fingolimod (0.5 mg daily) are detailed in Table S13 (see the Electronic Supplementary Material). Annual treatment discontinuation rates (Tables 2 and S14) and AE incidence rates (Table S15), including serious AEs other than PML, for natalizumab and fingolimod were extracted from published trials [20,21,22]. Annual discontinuation rates reflected all-cause discontinuation and were estimated from the intention-to-treat (ITT) populations of the pivotal studies for natalizumab and fingolimod (see Table S14 for additional details). PML incidence and fatality rates were taken from recent literature (Table S15). In addition to annual discontinuation rates, treatment stopping rules for progression to EDSS ≥ 7.0 and for conversion to SPMS were assumed.
After discontinuation of natalizumab or fingolimod and before conversion to SPMS, the model used the EDSS transition probability matrix from the BCMS database [8] and ARRs by EDSS from the placebo arm of the ITT population from AFFIRM (as in previous economic evaluations) [8, 19]. Data from the London Ontario MS database [7] and from previous cost-effectiveness analyses [19] were used for conversion to SPMS and for EDSS worsening and ARRs in SPMS (Table S16).
A constant standardised mortality ratio (SMR) for patients with MS (SMR = 2.88 [95% confidence interval (CI) 2.71–3.06]) [28] was applied multiplicatively to age- and sex-specific general population all-cause mortality probabilities from the UK [29]. Alternative mortality ratios by disease severity from an older study [30] were considered in scenario analysis.
2.3.2.3 Cost and Utility Data from the MS Burden-of-Illness Survey
Data from participants in a 2015 UK MS burden-of-illness survey [4, 31] were used to estimate direct and indirect costs and utility values (including those associated with relapses) by EDSS in RRMS and SPMS (Table 3 and Table S17 [see the Electronic Supplementary Material]). Indirect costs include short- and long-term absence, invalidity, and early retirement. A regression analysis was used to adjust the raw societal cost estimates by EDSS levels for differences between RRMS and SPMS, and indirect costs were estimated by subtracting the direct costs from the total societal costs [31]. Indirect costs were used in scenario analysis considering a societal perspective. UK-specific cost and utility estimates from a prior 2005 survey [9] were considered in scenario analysis (Table S18).
2.3.2.4 Other Cost and Utility Data
Annual acquisition costs for natalizumab and fingolimod were based on UK list prices [23] [Table 2 and Table S13 (see the Electronic Supplementary Material)]. Annual administration and monitoring costs, based on treatment-specific resource utilisation frequencies (e.g. physician visits, infusions, MRI scans, and lab tests), were estimated from costing templates, assumptions, and standard UK unit costs [24,25,26] (Table 2 and Table S13). Because fingolimod is covered by a confidential patient access scheme (PAS) in the UK [32], percentage reductions for the fingolimod acquisition price were considered in scenario analysis.
Direct costs and utility decrements per AE, including PML, were derived from estimates and assumptions obtained from the literature, previous HTA submissions for DMTs in RRMS, standard UK unit costs, and clinical experts (Table 2 [weighted outcomes] and Table S15 [detailed outcomes]). Caregiver disutilities by EDSS from a previous analysis [18] were used in scenario analysis considering a societal perspective (Table S17).
2.3.3 Analysis of Uncertainty
The impact of uncertainty in the base-case parameter values on the results of the cost-effectiveness analysis was assessed through one-way and probabilistic sensitivity analyses. The appropriate probability distributions for each parameter type were specified in accordance with best practices [33, 34] (see detailed table notes for specific distributions and uncertainty parameters). The one-way sensitivity analysis considered individual parameters at the lower and upper bounds of their 95% CIs, while the probabilistic sensitivity analysis sampled all parameters jointly from their respective probability distributions over 10,000 iterations. Additional deterministic scenarios were considered to evaluate the impact of alternative model settings, approaches to using the MSBase results, treatment discontinuation, and other data sources. Due to the limitations of interpreting negative incremental cost-effectiveness ratios, results were presented using a net monetary benefit (NMB) outcome measure assuming a willingness-to-pay threshold of £30,000 per quality-adjusted life-year (QALY) gained [17].
2.3.4 Validation
Cost-effectiveness model validation was performed in alignment with best practices, which emphasize the face validity of the modelling approach and data sources, the internal validity of the model programming and predicted outcomes, and the external validity of the predicted outcomes compared with other published studies [35, 36]. The appropriateness of our approach and data sources was supported by extensive modelling and HTA precedent in MS [10, 18] and the demonstrated clinical relevance of the MSBase registry [37]. The internal validity of the model was evaluated through extreme value testing and a comprehensive review of model logic and calculations by experts not involved in model development (results not shown). Finally, external validity was assessed by comparing our cost-effectiveness results with previously published analyses relying on trial-based efficacy values to compare natalizumab and fingolimod [38,39,40,41,42,43].
3 Results
3.1 Comparative Effectiveness
3.1.1 Patient Characteristics
Among 3935 patients with RRMS in the MSBase registry meeting the study criteria, 1135 switched to natalizumab, 907 switched to fingolimod, and 1893 switched to another BRACETD (Fig. 1). Table S1 in the Electronic Supplementary Material presents the raw unmatched patient characteristics. The three-way-multinomial-propensity-score matching algorithm [13] accounting for potential differences in baseline characteristics identified 897 matched patients in each cohort for inclusion in the analysis.
After matching, baseline characteristics were well balanced among treatment cohorts (Table 1). For patients switching to natalizumab, fingolimod, or within BRACETD, mean [standard deviation (SD)] post-index follow-up times were 2.56 (1.71), 2.05 (1.27), and 1.99 (1.52) years, respectively. Before switching, nearly all patients in each matched cohort were being treated with either interferon-based therapies (72.2–81.9%) or glatiramer acetate (15.8–25.1%) (Table S2). Among those switching within BRACETD, 78.9% continued to receive one of these therapies.
3.1.2 Relapses
The ARR for patients switching to natalizumab was significantly reduced compared with patients switching to fingolimod (RR = 0.70 [95% CI 0.62–0.79]) or switching within BRACETD (RR = 0.64 [95% CI 0.57–0.72]). In contrast, the reduction in ARR for those switching to fingolimod compared with those switching within BRACETD (RR = 0.91 [95% CI 0.81–1.03]) was not statistically significant (Fig. 3a).
Comparative effectiveness analysis results for natalizumab and fingolimod compared with BRACETD. ARR annualised relapse rate, BRACETD interferon-based therapies, glatiramer acetate, dimethyl fumarate, and teriflunomide, CDI6M 6-month–confirmed disability improvement, CDW6M 6-month–confirmed disability worsening, CI confidence interval, HR hazard ratio, RR risk ratio. Note: The comparative effectiveness outcomes for ARR (panel a), CDW6M (panel c), and CDI6M (panel d) for natalizumab and fingolimod compared with BRACETD were used in the base-case cost-effectiveness analysis. The time-to-first relapse outcomes (panel b) were not used in the cost-effectiveness analysis
In the time-to-first-relapse analysis, significant improvements were observed for patients switching to natalizumab (HR = 0.65 [95% CI 0.56–0.76]) and patients switching to fingolimod (HR = 0.86 [95% CI 0.74–0.99]) compared with those switching within BRACETD. Switching to natalizumab was also associated with an improved time to first relapse compared with switching to fingolimod (HR = 0.76 [95% CI 0.65–0.89]) (Fig. 3b). Tables S4–S7 in the Electronic Supplementary Material further describe the relapse analyses, including data maturity and results for the unmatched MSBase cohorts.
The impact of switching to natalizumab or fingolimod on ARRs in RRMS was also reflected in treatment-specific ARRs by EDSS estimated for use in the cost-effectiveness analysis (Tables S11 and S12). ARRs by EDSS score were also estimated for the unmatched MSBase cohorts (estimates not shown) for use in cost-effectiveness scenario analysis.
3.1.3 Disability Worsening and Improvement
The rates of CDW6M for patients in all three groups were not statistically significantly different from each other (Fig. 3c). The rate of CDI6M for patients switching to natalizumab increased significantly compared with that for those switching to fingolimod (HR = 1.28 [95% CI 1.01–1.62]) or switching within BRACETD (HR = 1.67 [95% CI 1.30–2.15]); the increase in the rate of CDI6M for patients switching to fingolimod was not significant compared with that for those switching within BRACETD (HR = 1.30 [95% CI 0.99–1.72]) (Fig. 3d). Tables S4–S7 in the Electronic Supplementary Material further describe the disability worsening and improvement analyses, including 3-month-confirmation results, data maturity, and results for the unmatched MSBase cohorts.
The impact of switching to natalizumab or fingolimod on annual probabilities of transitioning between EDSS scores in RRMS also was reflected in treatment-specific EDSS transition probability matrices estimated for use in the cost-effectiveness analysis (Tables S11 and S12). EDSS transition probability matrices also were estimated for the unmatched MSBase cohorts (estimates not shown) for use in cost-effectiveness scenario analysis.
3.2 Cost-Effectiveness
3.2.1 Base-Case Analysis
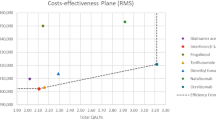
The clinical benefits observed in the comparative effectiveness analysis translated to the base-case cost-effectiveness results, where switching to natalizumab versus switching to fingolimod was predicted to lead to fewer lifetime relapses (13.25 vs. 14.27 [undiscounted]) and higher QALYs (7.87 vs. 7.42 [discounted]) (Table 4). The base-case cost-effectiveness analysis assumed that the only event impacting mortality differently between treatments was the occurrence of PML. Despite both treatments having been associated with PML, the relatively higher incidence of this AE for natalizumab led to the difference in total life-years between the treatments (Table 4). While natalizumab was associated with higher lifetime treatment costs than fingolimod (£3765 higher per patient) because of a longer time on treatment (5.56 vs. 4.31 years), the impact on disability accumulation (Fig. S2, see the Electronic Supplementary Material) and relapses led to lower disease-management and relapse costs for natalizumab versus fingolimod (£24,608 lower per patient) that more than offset the additional treatment costs (Table 4). As a result, natalizumab, in pharmacoeconomic terminology, dominated fingolimod, with higher predicted lifetime QALYs and lower predicted lifetime costs, in the base-case cost-effectiveness analysis (Table 4). At a willingness-to-pay threshold of £30,000 per QALY gained, the base-case NMB was estimated at £34,430 (Table 4).
3.2.2 Sensitivity and Scenario Analyses
In one-way and probabilistic sensitivity analyses, natalizumab remained dominant over fingolimod for all parameters varied (Fig. 4a) and for 99% of the 10,000 Monte-Carlo simulations (Fig. 4b), respectively. The incremental costs and QALYs for the one-way sensitivity analysis are presented in Table S19 (see the Electronic Supplementary Material).
One-way and probabilistic sensitivity analysis results for cost-effectiveness analysis. BRACETD interferon-based therapies, glatiramer acetate, dimethyl fumarate, and teriflunomide, EDSS Expanded Disability Status Scale, NMB net monetary benefit, OWSA one-way sensitivity analysis, PSA probabilistic sensitivity analysis, QALY quality-adjusted life-year, SPMS secondary progressive multiple sclerosis, WTP willingness-to-pay. Note: For all parameters varied in the OWSA (panel a), natalizumab remained dominant compared with fingolimod. The NMB outcomes were estimated using a WTP threshold of £30,000 per QALY gained. The PSA results are presented in panel b
Because fingolimod is covered by a confidential PAS in the UK [32], percentage discounts on the fingolimod list acquisition price were considered in scenario analysis. Natalizumab remained dominant with up to a 25.2% discount on the fingolimod list price and remained cost-effective at willingness-to-pay thresholds of £20,000 per QALY gained and £30,000 per QALY gained with up to 36.2 and 41.7% fingolimod discounts, respectively (Fig. S3).
Additional scenario analyses explored the impact of alternative model settings, approaches to using the MSBase results, discontinuation assumptions, and data sources. In all scenarios, natalizumab remained dominant compared to fingolimod (Table S20). Of note, shorter time horizons and higher annual discounting rates yielded lower incremental QALYs and lower cost savings, while a societal perspective led to higher incremental QALYs and greater cost savings. For the MSBase scenarios, using data from the unmatched cohorts had little impact on the cost-effectiveness results (< £1000 [0.05] difference in incremental costs [QALYs]), while different approaches to modelling treatment effectiveness (3-month disability confirmation, comparative effectiveness-adjusted vs. treatment-specific transition matrices and ARRs) led to larger differences in incremental outcomes. Scenarios considering equal treatment discontinuation rates led to lower incremental QALYs but higher cost savings. Increasing the discontinuation rate of natalizumab by 30% (8.1%) and decreasing the fingolimod one by 30% (7.2%) led to lower incremental QALYs and higher cost savings. Assuming a post-discontinuation treatment cost of £10,000 per year (the approximate annual cost of returning to BRACETD therapy) increased cost savings. Finally, using cost and utility data from the 2005 burden-of-illness survey and considering alternative mortality data by EDSS both led to higher incremental QALYs but lower cost savings (Table S20).
4 Discussion
This study is the first to use real-world effectiveness data in an economic evaluation of DMT escalation options for patients with HA-RRMS. Previously, economic evaluations comparing natalizumab with fingolimod have relied on trial-based efficacy estimates [10, 38, 40, 44]. Furthermore, the integrated approach to analysing comparative effectiveness and cost-effectiveness represents a step forward for the real-world economic evaluation of DMTs for MS and could serve as a model for the use of RWD in economic evaluations.
We conducted a three-way-multinomial-propensity-score–matched analysis to estimate impacts on relapse and disability with natalizumab and fingolimod in this population (Fig. 3). Switching to natalizumab reduced ARR by 30% and increased CDI6M rate by 28% compared with switching to fingolimod. When compared to switching to another BRACETD, switching to natalizumab reduced ARR by 36% and increased CDI6M rate by 67% (all outcomes above were statistically significant). Switching to fingolimod compared with switching to another BRACETD reduced ARR by 9% and increased CDI6M rate by 30% (although neither endpoint reached statistical significance). Time-to-first-relapse results directionally aligned with the ARR results. Conversely, no significant differences on CDW6M were observed among treatments. In the cost-effectiveness analysis based on these RWD estimates, natalizumab dominated fingolimod, leading to 0.453 higher QALYs and £20,843 lower per-patient total costs over a lifetime horizon (equating to a NMB of £34,430 at a willingness-to-pay threshold of £30,000 per QALY gained) (Table 4). These results were maintained across one-way and probabilistic sensitivity analyses and extensive scenario analyses (Fig. 4 and Table S20).
The comparative effectiveness analysis echoes findings from recent trial-based indirect treatment comparisons [45] and other RWD studies [11, 12, 46, 47] that natalizumab is an effective treatment for this population, with reduced relapse rates and disability improvement as key differentiators from fingolimod and BRACETD. Use of propensity-score matching ensured comparability among three treatment cohorts and allowed the use of the results in the cost-effectiveness analysis to present results comparing natalizumab and fingolimod. The higher effectiveness of natalizumab when compared with fingolimod supports previous findings that natalizumab is more effective than fingolimod among previously treated patients with higher baseline disease activity [48, 49].
The lack of significance in the comparative effectiveness results for fingolimod compared with BRACETD on ARR seemingly contradicts some previously published RWD analyses [50, 51]. The main difference between these studies is that the BRACETD-to-BRACETD switch group of the current study had 15.3% of patients switching to dimethyl fumarate, shown in some studies to have efficacy that is more similar to fingolimod than to other BRACETD therapies [52, 53].
Our cost-effectiveness results are broadly aligned with the results of other cost-effectiveness publications in the literature comparing natalizumab to fingolimod. Of seven publications comparing only these two treatments, natalizumab dominated fingolimod in four studies conducted across different countries (Sweden, the United States, and Colombia) and using different outcome measures (QALY, relapses, and disease progression) [38, 39, 42, 43]. In another study, natalizumab was considered cost-effective in the general RRMS population and dominant in the rapidly evolving severe (RES) sub-population [41]. One UK-based study also focusing on the RES sub-population used a discrete event simulation instead of a Markov model and found natalizumab to be cost-effective in the deterministic analysis at list price [40]. Finally, a study based in Iran concluded that fingolimod was cost-effective versus natalizumab; however, this study did not use clinical trial or registry data and used instead a census method to estimate outcomes for 81 patients with MS in one medical centre [54]. While the results of our study are largely aligned with other literature, care must be exercised when comparing different cost-effectiveness analyses. Costs, including drug costs and overall healthcare costs, tend to vary significantly between countries. The current study is the first to be based on effectiveness data from a large real-world registry, and this and other differences in data sources or outcome measures may also lead to different results. A final potential source of variation relates to the model structure, although the current model is similar to the majority of modelling approaches in MS in its use of EDSS health states first proposed 2 decades ago in the School of Health and Related Research (ScHARR) model [55].
The results and conclusions of this study are subject to the limitations typical of RWD analyses and economic evaluations. While propensity-score matching accounted for known differences between MSBase cohorts, bias due to unmeasured confounders (e.g. MRI activity) may remain. While the follow-up periods for the MSBase cohorts (means of 2–3 years, and variable among cohorts) were longer than the duration of clinical trials in RRMS (1–2 years), estimation of clinical and economic outcomes in the cost-effectiveness analysis still required significant extrapolation of the clinical benefits suggested by the MSBase results. There were differences in outcome definitions and confirmation requirements for EDSS changes between the MSBase time-to-event and transition matrix analyses, although scenario analyses investigated the impact of alternative MSBase settings on the cost-effectiveness results. Some data required to populate the economic model, such as AEs, discontinuation rates, and post-discontinuation ARRs by EDSS, were derived from the ITT populations of pivotal clinical trials, which were not restricted to patients who had failed a prior BRACETD treatment. The decision to estimate these model inputs from the ITT populations was driven by data availability, but may reduce the external validity of the model. Additionally, because of sample limitations within the MSBase registry at the time of the analysis, newer DMTs for RRMS (e.g. alemtuzumab, cladribine, and ocrelizumab) were not included in this study. Emerging RWD on these comparators [56, 57] may contribute to future evaluations of DMT escalation alternatives.
Finally, the clinical comparative effectiveness results from the MSBase analysis were sourced from an international registry with more than 130 different centres across 39 countries. This increases the applicability of the results to multiple geographies, especially because they reflect real-world clinical practice. Nevertheless, care should be taken because clinical practice, patient characteristics, and other factors may influence clinical outcomes. Conversely, results of a cost-effectiveness analysis often are country specific because inputs such as treatment and other healthcare costs and mortality data, among others, may vary significantly between countries. Adaptations of the current model to Spain and Italy [58, 59] have demonstrated how incremental costs and QALYs can differ across settings even when using the same underlying modelling approach. Therefore, extrapolating the economic implications to settings outside the UK would require additional analyses.
5 Conclusion
Overall, our results suggest that switching to natalizumab improves clinical and economic outcomes relative to switching to fingolimod in patients with HA-RRMS with inadequate response to BRACETD from a UK payer perspective. By relying on real-world effectiveness data from patients treated with natalizumab and fingolimod, this study provides improved external validity relative to trial-based analyses and offers additional evidence of natalizumab’s clinical and economic value as a treatment option for RRMS. These results complement the existing body of real-world evidence that should be considered when making individualised treatment decisions, taking into account the available therapies’ effectiveness and safety profiles and their impact on healthcare system resources.
Data Availability
The clinical data for this study was obtained under a licence agreement with MSBase (http://www.msbase.org). However, no patient-level data were disclosed as part of the study. Therefore, all data relevant to the study has been presented in the article and Supplementary Materials.
Change history
06 January 2022
The word document with the supplementary information has been corrected.
References
Butzkueven H, Chapman J, Cristiano E, et al. MSBase: an international, online registry and platform for collaborative outcomes research in multiple sclerosis. Mult Scler. 2006;12(6):769–74.
Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129(Pt 3):606–16.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52.
Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123–36.
Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354:i3518.
Pardo G, Jones DE. The sequence of disease-modifying therapies in relapsing multiple sclerosis: safety and immunologic considerations. J Neurol. 2017;264(12):2351–74.
Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914–29.
Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4(1):e004073.
Kobelt G, Berg J, Lindgren P, et al. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2006;77(8):918–26.
Guo S, Pelligra C, Saint-Laurent Thibault C, et al. Cost-effectiveness analyses in multiple sclerosis: a review of modelling approaches. Pharmacoeconomics. 2014;32(6):559–72.
Kalincik T, Horakova D, Spelman T, et al. Switch to natalizumab versus fingolimod in active relapsing–remitting multiple sclerosis. Ann Neurol. 2015;77(3):425–35.
Spelman T, Kalincik T, Zhang A, et al. Comparative efficacy of switching to natalizumab in active multiple sclerosis. Ann Clin Transl Neurol. 2015;2(4):373–87.
Rassen JA, Shelat AA, Franklin JM, et al. Matching by propensity score in cohort studies with three treatment groups. Epidemiology. 2013;24(3):401–9.
Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;31(122):552–68.
European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-multiple-sclerosis. Accessed 31 Mar 2015.
Craig BA, Sendi PP. Estimation of the transition matrix of a discrete-time Markov chain. Health Econ. 2002;11(1):33–42.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. https://www.nice.org.uk/process/pmg9/. Accessed 31 Jan 2018.
Gani R, Giovannoni G, Bates D, et al. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26(7):617–27.
Biogen Idec UK. Natalizumab (Tysabri) for the treatment of adults with highly active relapsing remitting multiple sclerosis. Single technology appraisal (STA) submission to the National Institute for Health and Clinical Excellence. NICE manufacturer’s submission. 2007. https://www.nice.org.uk/guidance/ta127/history.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15.
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
British National Formulary. 79th edition. 2020. https://www.medicinescomplete.com/mc/bnf/current/. Accessed 13 Oct 2020.
Curtis L, Burns A. Unit costs of health and social care 2019. Personal Social Services Research Unit, University of Kent, Canterbury. 2019. https://doi.org/10.22024/UniKent/01.02.79286.
Nottingham University Hospitals. High cost tests referred to other labs. Feb 9. https://www.nuh.nhs.uk/staff-area/microbiology/turnaround-times/high-cost-referred-tests/. Accessed 9 Feb 2017.
National Health Service Reference Costs. 2018–2019. https://www.england.nhs.uk/wp-content/uploads/2020/08/National_Cost_Collection_2018-19_A_Guide_to_using_the_data.pdf. Accessed 15 Oct 2020.
Office for National Statistics. Consumer Price Index. 2020. https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/d7bz/mm23. Accessed 15 Oct 2020.
Kingwell E, van der Kop M, Zhao Y, et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J Neurol Neurosurg Psychiatry. 2012;83(1):61–6.
Office for National Statistics. National life tables, United Kingdom, 2014–2016. 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables. Accessed 30 Jan 2018.
Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med. 1997;29(2):101–6.
Thompson A, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: results for the United Kingdom. Mult Scler. 2017;23(2_suppl):204–16.
National Institute for Health and Care Excellence. List of technologies with approved Patient Access Schemes. 2017. https://www.nice.org.uk/about/what-we-do/patient-access-schemes-liaison-unit/list-of-technologies-with-approved-patient-access-schemes. Accessed 5 Sept 2017.
Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Mak. 2012;32(5):722–32.
Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Value Health. 2012;15(6):843–50.
Vemer P, Corro Ramos I, van Voorn GA, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61.
Kalincik T, Butzkueven H. The MSBase registry: informing clinical practice. Mult Scler. 2019;25(14):1828–34.
Bozkaya D, Livingston T, Migliaccio-Walle K, et al. The cost-effectiveness of disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis. J Med Econ. 2017;20(3):297–302.
Lasalvia P, Hernández F, Castañeda-Cardona C, et al. Cost-effectiveness of natalizumab compared with fingolimod for relapsing-remitting multiple sclerosis treatment in Colombia. Value Health Reg Issues. 2020;23:13–8.
Montgomery SM, Maruszczak MJ, Slater D, et al. A discrete event simulation to model the cost-utility of fingolimod and natalizumab in rapidly evolving severe relapsing-remitting multiple sclerosis in the UK. J Med Econ. 2017;20(5):474–82.
O’Day K, Meyer K, Stafkey-Mailey D, et al. Cost-effectiveness of natalizumab vs fingolimod for the treatment of relapsing-remitting multiple sclerosis: analyses in Sweden. J Med Econ. 2015;18(4):295–302.
Alexopoulos S, Deniz B, Walker A, et al. Economic evaluation of NATALIZUMAB versus fingolimod for the treatment of relapsing-remitting multiple sclerosis in Sweden. Poster (PND23). ISPOR. Washington, DC, USA; 2012.
O’Day K, Meyer K, Miller RM, et al. Cost-effectiveness of natalizumab versus fingolimod for the treatment of relapsing multiple sclerosis. J Med Econ. 2011;14(5):617–27.
Hettle R, Harty G, Wong SL. Cost-effectiveness of cladribine tablets, alemtuzumab, and natalizumab in the treatment of relapsing-remitting multiple sclerosis with high disease activity in England. J Med Econ. 2018;21(7):676–86.
McCool R, Wilson K, Arber M, et al. Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:55–61.
Lorscheider J, Benkert P, Lienert C, et al. Comparative analysis of natalizumab versus fingolimod as second-line treatment in relapsing-remitting multiple sclerosis. Mult Scler. 2018;24(6):777–85.
Prosperini L, Sacca F, Cordioli C, et al. Real-world effectiveness of natalizumab and fingolimod compared with self-injectable drugs in non-responders and in treatment-naive patients with multiple sclerosis. J Neurol. 2017;264(2):284–94.
Trojano M, Tintore M, Montalban X, et al. Treatment decisions in multiple sclerosis—insights from real-world observational studies. Nat Rev Neurol. 2017;13(2):105–18.
Kalincik T. Comparisons of therapies in different scenarios help complete the puzzle. Mult Scler. 2018;24(6):694–5.
He A, Spelman T, Jokubaitis V, et al. Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. JAMA Neurol. 2015;72(4):405–13.
Braune S, Lang M, Bergmann A, et al. Efficacy of fingolimod is superior to injectable disease modifying therapies in second-line therapy of relapsing remitting multiple sclerosis. J Neurol. 2016;263(2):327–33.
Braune S, Grimm S, van Hovell P, et al. Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: results from the German NeuroTransData registry. J Neurol. 2018;265(12):2980–92.
Kalincik T, Kubala Havrdova E, Horakova D, et al. Comparison of fingolimod, dimethyl fumarate and teriflunomide for multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(4):458–68.
Rezaee M, Izadi S, Keshavarz K, et al. Fingolimod versus natalizumab in patients with relapsing remitting multiple sclerosis: a cost-effectiveness and cost-utility study in Iran. J Med Econ. 2019;22(4):297–305.
Tappenden P, Chilcott J, O’Hagan A, et al. Cost effectiveness of beta interferons and glatiramer acetate in the management of multiple sclerosis. Sheffield: Centre for Bayesian Statistics in Health Economics, School of Health and Related Research (ScHARR); 2001.
Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16(4):271–81.
Kalincik T, Jokubaitis V, Spelman T, et al. Cladribine versus fingolimod, natalizumab and interferon beta for multiple sclerosis. Mult Scler. 2017;1:1352458517728812.
Herring W, Gould IG, Casamassima G, et al. A cost-effectiveness analysis using real-world data from the MSBase registry: comparing natalizumab to fingolimod in patients with inadequate response to disease-modifying therapies in relapsing-remitting multiple sclerosis in Italy. Value Health. 2018;21:S338.
Herring W, Gould IG, Ruiz L, et al. A cost-effectiveness analysis using real-world data from the MSBase registry: comparing natalizumab to fingolimod in patients with inadequate response to disease-modifying therapies in relapsing-remitting multiple sclerosis in Spain. Value Health. 2018;21:S338.
Acknowledgements
Thibaut Dort was an employee of Biogen at the time the study was conducted and the manuscript drafted. This manuscript is dedicated to his memory, as he passed away in April 2019, before this manuscript was completed. The authors also would like to acknowledge the contributions of the following individuals: Ryan Ziemiecki of RTI Health Solutions provided guidance on the design of the comparative effectiveness analysis; Karyn Hede, Kate Lothman, and Emily Gill of RTI Health Solutions provided editorial and graphical support during the preparation of the manuscript.
MSBase Investigators T. Spelman1, E. Havrdova2, D. Horakova3, M. Trojano4, G. De Luca5, A. Lugaresi6,7, G. Izquierdo8, P. Grammond9, P. Duquette10, R. Alroughani11, E. Pucci12, F. Granella13, J. Lechner-Scott14, P. Sola15, D. Ferraro16, F. Grand’Maison17, M. Terzi18, C. Rozsa19, C. Boz20, R. Hupperts21, V. Van Pesch22, C. Oreja-Guevara23, A. van der Walt1, V. G. Jokubaitis1, T. Kalincik24, H. Butzkueven1
Affiliations
1. Department of Neuroscience, Central Clinical School Alfred Hospital, Monash University, Melbourne, VIC, Australia
2. Department of Neurology and Centre of Clinical Neuroscience, First Faculty of Medicine, General University Hospital and Charles University, Prague, Czech Republic
3. Department of Basic Medical Sciences, Neuroscience and Sense Organs, University of Bari, Bari, Italy
4. Multiple Sclerosis Centre, Neurology Unit, SS Annunziata Hospital, University “G. d’Annunzio”, Chieti-Pescara, Italy
5. IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy
6. Dipartimento di Scienze Biomediche e Neuromotorie, Università di Bologna, Bologna, Italy
7. Hospital Universitario Virgen Macarena, Seville, Spain
8. Centre de Réadaptation Déficience Physique Chaudière-Appalache, Levis, Canada
9. Hôpital Notre Dame, Montreal, Canada
10. Amiri Hospital, Kuwait City, Kuwait
11. Neurology Unit, ASUR Marche–AV3, Macerata, Italy
12. University of Parma, Parma, Italy
13. John Hunter Hospital, Newcastle, Australia
14. Azienda Ospedaliero Universitaria Policlinico/OCB, Neurology Unit, Modena, Italy
15. Department of Biomedical, Metabolic and Neurosciences, University of Modena and Reggio Emilia, Modena, Italy
16. Neuro Rive-Sud, Hôpital Charles LeMoyne, Longueuil, Canada
17. Mayis University, Samsun, Turkey
18. Jahn Ferenc Teaching Hospital, Budapest, Hungary
19. Karadeniz Technical University, Trabzon, Turkey
20. Zuyderland Medical Center, Sittard, The Netherlands
21. Cliniques Universitaires Saint-Luc, Brussels, Belgium
22. Hospital ClínicoSan Carlos, Madrid, Spain
23. CORe, Department of Medicine, University of Melbourne, and Department of Neurology, Royal Melbourne Hospital, Melbourne, Australia
Funding
This research was supported by Biogen International GmbH (Baar, Switzerland); MSBase receives general financial support from Biogen, Genzyme, Merck (MSD), Merck Serono, Novartis, Roche, and Teva.
Author information
Authors and Affiliations
Consortia
Contributions
The comparative effectiveness study was conducted by MSBase. TS and HB conducted and supervised the analysis of the MSBase registry data, respectively. The remaining MSBase-affiliated authors contributed to the collection and interpretation of the data. The cost-effectiveness study was conducted by RTI Health Solutions. WH and YZ developed the cost-effectiveness model and contributed to the interpretation of analytical results. IP contributed to the design of the cost-effectiveness model and the interpretation of analytical results. MT provided the country-specific non-clinical inputs for the cost-effectiveness model. CA, TD, UF, and RH supervised the study for both the clinical and cost-effectiveness analysis. Both MSBase and RTI Health Solutions operated under separate research contracts with Biogen and were funded by Biogen. All authors reviewed the data and contributed to the final interpretation of results.
Corresponding author
Ethics declarations
Conflict of interest
Timothy Spelman is a statistical contractor for the MSBase Foundation and has received compensation for serving on advisory boards for Biogen and speaker honoraria from Novartis and Roche. William L. Herring, Yuanhui Zhang, and Isobel Pearson are employees of RTI Health Solutions, an independent non-profit research organisation, which received funding pursuant to a contract with Biogen. Michael Tempest, Ulrich Freudensprung, Carlos Acosta, and Robert Hyde are employees of Biogen and hold shares or stocks from Biogen as part of their remuneration. Thibaut Dort was an employee and shareholder of Biogen at the time of his passing. Eva Havrdova has received honoraria and research support from Biogen, Merck Serono, Novartis, Roche, and Teva; has served in advisory boards for Actelion, Biogen, Celgene, Merck Serono, Novartis, and Sanofi Genzyme; and has been supported by the Czech Ministry of Education research project PROGRES Q27/LF1. Dana Horakova has received speaker honoraria and consulting fees from Biogen, Merck, Novartis, Roche, Sanofi Genzyme, and Teva and has received support for research activities from Biogen and the Czech Ministry of Education (project Progres Q27/LF1). Maria Trojano has served on scientific advisory boards for Biogen, Novartis, Roche, and Merck; has received speaker honoraria from Biogen, Sanofi, Merck, Roche, Teva, and Novartis; and has received research grants for her Department from Biogen, Merck, Roche, and Novartis. Giovanna De Luca has served on scientific advisory boards for Merck, Biogen, Novartis, Roche, and Sanofi Genzyme and has received funding for travel and speaker honoraria from Biogen, Merck, Novartis, Sanofi Genzyme, and Roche. Alessandra Lugaresi has received personal compensation for consulting, serving on a scientific advisory board, speaking or other activities from Biogen, Merck Serono, Mylan, Novartis, Roche, Sanofi/Genzyme, and Teva. Her institutions have received research grants from Novartis. Guillermo Izquierdo has received speaking honoraria from Almirall, Biogen, Merck, Novartis, Roche, Sanofi, and Teva. Pierre Grammond has served in advisory boards for Novartis, EMD Serono, Roche, Biogen, Sanofi Genzyme, and Pendopharm and has received grant support from Genzyme and Roche; his institution has also received research grants from Biogen, Sanofi Genzyme, and EMD Serono. Pierre Duquette has received support for organised continuing medical education activities and travel fees to attend advisory meetings from Biogen, EMD Serono, Genzyme, and Novartis. Raed Alroughani has received honoraria as a speaker and for serving on scientific advisory boards from Bayer, Biogen, GSK, Merck, Novartis, Roche, Sanofi, and Genzyme. Eugenio Pucci has received travel grants from Roche, Novartis, Merck, Genzyme-Sanofi, Biogen, and Teva and received equipment from Associazione Marchigiana Sclerosi Multipla e altre malattie neurologiche. Franco Granella has received research funding from Biogen and Sanofi Genzyme; fees for advisory boards and speaker honoraria from Biogen, Merck Serono, Novartis, Roche, and Sanofi Genzyme; and travel funding from Biogen, Merck Serono, Roche, and Sanofi Genzyme. Jeannette Lechner-Scott has accepted travel compensation from Novartis, Biogen, and Merck Serono; her institution receives the honoraria for talks and advisory board commitments as well as research grants from Biogen Merck, Roche, TEVA, and Novartis. Patrizia Sola has served on scientific advisory boards for Biogen and Teva; has received funding for travel and speaker honoraria from Bayer, Biogen, Merck, Novartis, Sanofi Genzyme, and Teva; and has received research grants for her institution from Bayer, Biogen, Merck, Novartis, Sanofi, and Teva. Diana Ferraro has received travel funding or speaker honoraria from Merck, Biogen, Sanofi, Novartis, Teva, and Roche and has served on advisory boards for Biogen, Merck, Novartis, Sanofi, and Roche. Francois Grand’Maison has received honoraria or research funding from Biogen, Genzyme, Mitsubishi, Novartis, Ono Pharmaceuticals, and Teva Neuroscience. Murat Terzi has received travel grants from Merck & Co., Novartis, Bayer-Schering, and Teva Pharmaceutical Industries and has participated in clinical trials by Sanofi-Aventis, Roche, and Novartis. Csilla Rozsa has received speakers’ honoraria from Biogen, Merck Serono, Roche, and Sanofi Genzyme; travel grants from Biogen, Merck, and Sanofi Genzyme; and fees for advisory boards from Biogen, Merck Serono, Novartis, and Teva. Cavit Boz has received conference travel support from Biogen, Novartis, Bayer-Schering, Merck, and Teva. Raymond Hupperts has received nurse support from Merck and Sanofi and has served on advisory boards for Sanofi, Biogen, and Roche. Vincent Van Pesch has received travel grants from Merck, Biogen, Sanofi, Bristol Meyer Squibb, Almirall, and Roche. His institution has received research grants and consultancy fees from Roche, Biogen, Sanofi, Bristol Meyer Squibb, Merck, Almirall, and Novartis Pharma. Celia Oreja-Guevara has received honoraria for speaking and consulting fees from Biogen, Celgene, Alexion, Merck, Novartis, Roche, Sanofi Genzyme, and Teva. Anneke van der Walt is an employee of Monash University and has accepted travel compensation from Merck, Biogen, and Roche; she has received honoraria for talks, steering committee activities, and research grants from Roche, Merck, Biogen, and Novartis. Vilija Jokubaitis is an employee of Monash University; she has accepted travel compensation from Biogen, Merck, and Roche and speakers’ honoraria from Biogen and Roche. Tomas Kalincik has served on scientific advisory boards for Roche, Sanofi Genzyme, Novartis, Merck, and Biogen and a steering committee for the Brain Atrophy Initiative supported by Sanofi Genzyme; has received conference travel support and/or speaker honoraria from WebMD Global, Novartis, Biogen, Sanofi Genzyme, Teva, BioCSL, and Merck; and has received research support from Biogen. Helmut Butzkueven is an employee of Monash University and has accepted travel compensation from Merck, Biogen, and Roche; his institution receives honoraria for talks, steering committee activities, and research grants from Roche, Merck, Biogen, Genzyme, Novartis, and Medday Pharma.
Ethics approval and consent
Informed consent from all patients according to local laws is required for participation in MSBase, and the project has received human research ethics committee approval or an exemption at each contributing centre.
Additional information
The original Online version of this article was revised: The author names in track changes was published inadvertently in the supplementary word document.
The MSBase Investigators are listed in Acknowledgements.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Spelman, T., Herring, W.L., Zhang, Y. et al. Comparative Effectiveness and Cost-Effectiveness of Natalizumab and Fingolimod in Patients with Inadequate Response to Disease-Modifying Therapies in Relapsing-Remitting Multiple Sclerosis in the United Kingdom. PharmacoEconomics 40, 323–339 (2022). https://doi.org/10.1007/s40273-021-01106-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-021-01106-6