Abstract
Objective
The aim was to project the health and economic outcomes of cardiovascular disease (CVD) among people with type 2 diabetes from Australian public healthcare and societal perspectives over the next decade.
Methods
A dynamic multistate model with yearly cycles was developed to project cardiovascular events among Australians with type 2 diabetes aged 40–89 years from 2022 to 2031. CVD risk (myocardial infarction [MI] and stroke) in the type 2 diabetes population was estimated using the 2013 pooled cohort equation, and recurrent cardiovascular event rates in the type 2 diabetes with established CVD population were obtained from the global Reduction of Atherothrombosis for Continued Health (REACH) registry. Costs and utilities were derived from published sources. Outcomes included fatal and non-fatal MI and stroke, years of life lived, quality-adjusted life years (QALYs), total healthcare costs, and total productivity losses. The annual discount rate was 5%, applied to outcomes and costs.
Results
Between 2022 and 2031, a total of 83,618 non-fatal MIs (95% uncertainty interval [UI] 83,170–84,053) and 58,774 non-fatal strokes (95% UI 58,458–59,013) were projected. Total years of life lived and QALYs (discounted) were projected to be 9,549,487 (95% UI 9,416,423–9,654,043) and 6,632,897 (95% UI 5,065,606–7,591,679), respectively. Total healthcare costs and total lost productivity costs (discounted) were projected to be 9.59 billion Australian dollars (AU$) (95% UI 1.90–30.45 billion) and AU$9.07 billion (95% UI 663.53 million–33.19 billion), respectively.
Conclusions
CVD in people with type 2 diabetes will substantially impact the Australian healthcare system and society over the next decade. Future work to investigate different strategies to optimize the control of risk factors for the prevention and treatment of CVD in type 2 diabetes in Australia is warranted.
Similar content being viewed by others
Cardiovascular disease (CVD) prevalence in type 2 diabetes is likely to increase. The current multistate dynamic economic model is the first to project the impact of CVD among Australians with type 2 diabetes, aged 40–89 years, from 2022 to 2031. Findings will serve as a foundation for future work to project the societal impacts of different strategies to mitigate the burden of CVD in type 2 diabetes. |
The chronic costs of CVD contributed to 58.17% (5.58 billion Australian dollars [AU$]) of the total healthcare cost, followed by acute costs (21.83%, AU$2.09 billion) and pharmacological treatment costs (20%, AU$1.92 billion). |
Morbidity contributed to 57.35% (AU$5.20 billion) of the total productivity loss cost, and premature mortality contributed to 42.65% (AU$3.87 billion) of the total productivity loss cost. |
1 Introduction
Diabetes is a significant risk factor for cardiovascular disease (CVD), with a two- to threefold higher risk than that in individuals without diabetes [1]. A systematic review, including more than 4 million individuals with type 2 diabetes, showed that CVD was responsible for 50.3% of all deaths globally [2]. Data from Australia estimated that 57% of people with diabetes have CVD, and nearly 65% of CVD-related deaths occur in people with prediabetes or diabetes [3, 4]. Likewise, in Europe and Canada, and despite advances in CVD prevention and treatment, studies report persistent excess death associated with diabetes [5,6,7,8,9].
Nonetheless, in the United States (US), a reduction in all-cause and CVD deaths was reported (23% and 40%) in people with diabetes between 1997 and 2006, suggesting improvement in CVD risk-factor control [10]. Similarly, in Australia, Harding et al. showed a significant decrease in all-cause and CVD deaths among Australians with type 2 diabetes between 1997 and 2010 [11]. Also, the incidence of stroke remained stable from 2010 to 2019, while the incidence of myocardial infarction (MI) decreased from 2012–2013 to 2018–2019 in Australians with type 2 diabetes [12]. Despite these encouraging trends and efforts in expanding evidence-based guidelines for managing CVD in people with type 2 diabetes [13, 14], CVD prevalence in type 2 diabetes is a burden that is likely to persist [4]. In Australia, for example, suboptimal management among type 2 diabetic people at risk of CVD has been reported [15,16,17], and treatment targets are not met [15,16,17,18].
The health burden associated with CVD in people with type 2 diabetes imposes a substantial economic concern on healthcare systems and on society. In Australia, the total direct costs for people with diabetes were 2353 Australian dollars (AU$) per person with normal weight and AU$3131 per person with obesity in 2016–2017 [19]. The Australian Institute of Health and Welfare (AIHW), for 2018–2019, reported that nearly AU$3 billion was spent on diabetes, representing 2.3% of total disease expenditure in Australia [20]. Furthermore, a recent modeling study estimated that the burden of CVD in the general Australian population over the next decade would lead to AU$61.89 billion in healthcare costs and AU$78.75 billion in indirect costs due to lost productivity [21]. This study, however, analyzed the whole Australian population and did not provide insights into the excess CVD burden in type 2 diabetes [21].
As the majority of CVD deaths in Australia occur in people with prediabetes or diabetes, prevalence rates of CVD outcomes are required to forecast the societal burden of CVD in type 2 diabetes. Therefore, we projected the health and economic burden of CVD among people with type 2 diabetes in Australia for each year from 2022 to 2031 using a dynamic multistate model, which allows for the movement of individuals into and out of the simulations and accounts for changes in mortality and migration as well as incident type 2 diabetes.
2 Methods
2.1 Model Structure and Description
After reviewing prior published studies on the burden of CVD complications due to type 2 diabetes [21], a two-stage dynamic multistate Markov model was constructed using Microsoft Excel to predict the societal burden of CVD among Australians with type 2 diabetes using yearly cycles.
The model covered a 10-year time horizon from 2022 to 2031, and the population of interest was people with type 2 diabetes aged 40–89 years, stratified according to age and sex [22]. Here, the population with type 2 diabetes was allowed to enter the model at the age of 40 years, assuming that at 40 years old the risk of CVD increases among the Australian population [23], and exits when they reach age 89 years, which nearly reflects the life expectancy in Australia (84.3 years in 2022) [24]. Overall, the model followed individuals for 10 years, until they died or until they reached 89 years of age in the model. This age range aligns with similar studies conducted on people with or at risk of CVD [21, 25, 26].
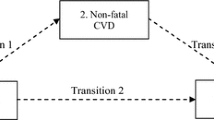
The use of prior published studies for cardiovascular complications in people with type 2 diabetes allowed the structuring of the model with relevant health states [21, 25, 26]. The model captured incident and recurrent non-fatal cardiovascular events (i.e., MI and stroke) and mortality (CVD and non-CVD causes) among people with type 2 diabetes, with and without established CVD. The model assumed that individuals aged 40–89 years with type 2 diabetes without prior CVD would enter the ‘Alive with type 2 diabetes without established CVD’ health state. Within this health state, individuals can have a non-fatal or fatal cardiovascular event and move to the ‘Alive with type 2 diabetes with established CVD’ or ‘Dead’ health state (Fig. 1). In the ‘Alive with type 2 diabetes with established CVD’ health state, the population could suffer a recurrent non-fatal CVD or die due to CVD or other causes (Fig. 1).
The main model outcomes were years of life lived, quality-adjusted life years (QALYs), direct healthcare costs, and indirect costs due to productivity losses. The outcomes were discounted at a rate of 5% annually starting in the second year of the model [27]. The study was conducted from Australian public healthcare and societal perspectives.
The model was developed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, US). The model adhered to the Australian methodological guidelines for conducting Health Technology Assessment [28], and the reporting followed the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) [29].
2.2 Model Population
The demographic profile of the model population was based on the Australian population obtained from the 2021 estimated resident population [22]. The prevalence and incidence of type 2 diabetes, stratified by age and sex, were derived from the National Diabetes Services Scheme (NDSS) (2019) [30]. Our model uses a dynamic population that reflects the movement of individuals into and out of the simulations and changes in mortality and migration, as well as incident type 2 diabetes.
2.2.1 Type 2 Diabetes with and Without Established Cardiovascular Disease Populations
The population was based on the Australian resident population in 2021 derived from the Australian Bureau of Statistics (ABS) [22] (electronic supplementary material [ESM] Table 1). The prevalence of type 2 diabetes, stratified by age and sex for the latest available year (2019) (ESM Table 2) was derived from the NDSS [30]. ESM Table 2 also shows the number of Australians with type 2 diabetes. Contemporary age- and sex-specific prevalence estimates of established CVD (non-fatal MI and stroke) for Australians were based on data from the general Australian population from the latest ABS data, 2017–2018 [31] (ESM Table 3). The population was then divided into people with and without prior CVD. Characteristics of both resultant cohorts are shown in ESM Table 4.
2.3 Cardiovascular Disease Risk in the Type 2 Diabetes Without Cardiovascular Disease Population
The risk of incident CVD among the type 2 diabetes without CVD population was calculated based on the 2013 Pooled Cohort Equation–Atherosclerotic Cardiovascular Disease (PCE-ASCVD) [32], which was designed to estimate the 10-year absolute rate of incident CVD. Risk estimates were extracted from the National Health Survey (NHS) (2011–2012) [33] and were calculated based on sex, age, smoking status, systolic blood pressure, diabetes status, hypertension treatment status, and total and high-density lipoprotein cholesterol levels. The 2013 PCE-ASCVD has been validated, and showed better performance in Australians with type 2 diabetes compared to other CVD risk prediction tools [34]. The NHS included 4205 Australians, of which 313 had type 2 diabetes and were aged between 40 and 90 years. Characteristics of the Australian population included in the PCE-ASCVD to estimate the CVD risk for the type 2 diabetes without CVD population are described in ESM Table 5. The next step was to derive sex- and age-specific average 10-year risk scores and convert these scores to annual probabilities. Annual event rates were plotted against the midpoints of each 10-year age group, and polynomial functions were used to model the values for every year of age (ESM Table 6). The PCE-ASCVD algorithm only estimates composite CVD outcomes (fatal and non-fatal combined); thus, to estimate the proportions of fatal and non-fatal MI and stroke events among Australians, Australian Institute of Health and Welfare data were used [35, 36]. This corresponded to 0.28 and 0.72 for fatal and non-fatal MI and 0.32 and 0.68 for fatal and non-fatal stroke, respectively. Type 2 diabetes without CVD captured the population's dynamic movement that considered predicted migration and mortality changes. Migration in people with type 2 diabetes and mortality data stratified by sex and single year of age were extracted from the 2017 ABS [37].
2.4 Cardiovascular Disease Risk in the Type 2 Diabetes with Established Cardiovascular Disease Population
To calculate the transition probabilities of recurrent fatal and non-fatal CVD in people with type 2 diabetes and established CVD, data from the Reduction of Atherothrombosis for Continued Health (REACH) registry were used, which includes 30,043 people with diabetes [38]. Cardiovascular risk rates from the REACH registry were only reported as overall rates. Thus, these were adjusted to single-age event probabilities via increasing or decreasing the cardiovascular risk from the median age in the REACH registry (72 years) as per age variations in cardiovascular mortality rates in Australia (thus assuming the effect of age on fatal and non-fatal CVD is equivalent). Fatal and non-fatal cardiovascular event rates for diabetes people can be seen in ESM Table 7, and the estimated transition probabilities are shown in ESM Table 8. To account for the non-CVD deaths in this cohort, we initially used the age- and sex-specific mortality rates for the general Australian population from the General Record of Incidence of Mortality 2019, as contemporary age- and sex-specific mortality rates for Australians with type 2 diabetes are lacking [39]. Then we adjusted these rates for people with type 2 diabetes based on data from a Danish diabetes registry that included over 400,000 individuals with type 2 diabetes from 1996 to 2016 [40]. To estimate the mortality rates by single year of age, mortality rates for the age group were first plotted against the midpoint age for that age group (e.g., 50 for the age group 45–54 years). Then polynomial functions were applied to model the mortality rates for age in single years (ESM Table 9).
2.5 The Incidence of Type 2 Diabetes
To account for the new cases of type 2 diabetes over time, incident type 2 diabetes cases were added each year for type 2 diabetes to the cohorts with and without CVD until 2031. Age- and sex-specific incidence rates of type 2 diabetes were obtained from the NDSS (2019) [30], and we assumed that the incidence rates would remain constant throughout the time horizon (ESM Table 10 shows the incidence rate of type 2 diabetes per 1000 persons). Because the NDSS does not quantify the proportion of people with incident type 2 diabetes with or without prior MI/stroke, published sources were used to estimate the ratios. Further descriptions are provided in ESM Table 10.
2.6 Utilities
To account for differences by age and sex, all utilities were adjusted to age- and sex-specific values reported by a cross-sectional study by McCaffrey et al., which included a sample of 2900 from the general Australian population [41]. The utility weight for type 2 diabetes (0.83) was derived from the ADVANCE trial, which included 978 Australians with type 2 diabetes [42]. The same study was used to obtain the weighted utilities of acute (at baseline) and chronic (at 2 years) CVD events. The utility score in people with type 2 diabetes who had acute MI was 0.80, and was 0.75 for acute stroke. The utility for chronic CVD (MI/stroke) was set at 0.81 with a weighted utility score decrement of 0.02. To calculate the composite utility within 12 months for the type 2 diabetes population who experience the first non-fatal event (MI/stroke), we applied 6 months of the ‘type 2 diabetes’ utility score, 3 months of the acute utility (MI/stroke), and 3 months of the chronic CVD utility. For the type 2 diabetes with established CVD population, acute utility scores were applied for 3 months, and chronic utilities were applied for the remaining 9 months. An example of age- and sex-specific utility for people in the type 2 diabetes population is represented in Box 1.
All utility data can be found in ESM Table 11.
2.8 Resources and Costs
2.8.1 Direct Healthcare Costs
Healthcare costs included acute costs, annual chronic costs, glucose-lowering therapies (GLTs) costs, and lipid-lowering therapies costs. As per the Royal Australian College of General Practitioners guideline [43], GLTs included metformin, sulfonylurea, dipeptidyl peptidase-4 inhibitors (DPP4i), sodium-glucose co-transporter 2 inhibitors (SGLTi), and glucagon-like peptide-1 receptor agonists (GLP-1 RA). Lipid-lowering therapies included statins, ezetimibe, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended for people with type 2 diabetes with or at risk of CVD [44]. The annual costs of metformin, other GLTs (non-metformin), statins, ezetimibe, and PCSK9 inhibitors were AU$56, AU$4551, AU$914, AU$277, and AU$6582, respectively (per person), obtained from the Pharmaceutical Benefits Scheme [45]. We assumed that all people would receive first-line metformin as per current Australian guidelines [43], while the proportions of other GLTs as second-line therapies were based on Morton et al. [46] (32% for DPP4i, 30% for SGLTi, 17% for sulfonylurea, and 6% for GLP-1 RA). We also assumed that once a non-fatal MI/stroke occurs, 95% of people would receive statins and 5% would receive non-statins [47].
Acute costs for CVD included non-fatal MI, non-fatal stroke, and death due to CVD. These were drawn from Australian hospital admission data (2018) and classified into Australian Refined Diagnosis-Related groups (Version 8, Round 21) [48], which were estimated to be AU$9029, AU$13,198, and AU$6928, respectively (per person). The chronic costs of non-fatal MI and stroke in Australians with type 2 diabetes were derived from published data by Clarke et al., which captured the sum of hospital inpatient and non-hospital mean costs using administrative data and excluded pharmaceutical costs [49]. The annual chronic costs of non-fatal MI and stroke in type 2 diabetes were AU$2656 and AU$5832, respectively (per person). The model considered that 50% of deaths occur in hospitals, and thus, death costs were only applied to 50% of deaths [50].
2.8.2 Lost Productivity
This included costs due to absenteeism, workforce drop-out, and loss of future earnings due to premature mortality, using the human capital approach [51]. Salary losses due to absenteeism were calculated as the average days off from work due to a non-fatal MI/stroke [52, 53] multiplied by the average daily salaries among Australians [54]. Average wages were adjusted for employment rates based on age- and sex-specific data in Australia for the full-time working population [55], with the retirement age in this study set at 67 years [56].
The effect of type 2 diabetes on workforce participation in Australia is minimal; therefore, we assumed that workforce participation is the same as in the general Australian population [57]. Salary losses due to workforce drop-out after CVD development were calculated based on the proportion of individuals not returning to work (9% due to MI and 38% due to stroke), as reported in previous studies [53, 58]. Loss of future earnings due to premature mortality was calculated by multiplying the remaining years left in the workforce or years left in the model, whichever came first, by age- and sex-specific salary levels and employment rates among Australians [55]. All costs were adjusted to 2022 AU$ using the Health Price Index (ESM Table 12) [59].
2.9 Sensitivity Analyses
Probabilistic sensitivity analyses (PSA) were conducted to quantify parameter uncertainties using 10,000 iterations of a Monte Carlo simulation. An uncertainty range of ±95% confidence interval (CI) was assigned to the base-case values of transition probabilities for the type 2 diabetes with and without CVD populations, and to the proportions of fatal and non-fatal MI and stroke that were used to estimate the transition probabilities in the type 2 diabetes with and without CVD populations. An uncertainty range of ± 95% CI was also used for the utilities and costs. The analysis was performed using @Risk-7.5® (Palisade Corporation, NY, US). Key input parameters and respective distributions are displayed in ESM Table 13. In addition to the PSA, multiple scenario analyses were conducted:
-
1.
Removing the age-related death trends from cardiovascular causes in the type 2 diabetes with established CVD population
-
2.
Varying the discount rate between 0, 3, 4, and 6%
-
3.
Removing the limit to the model time horizon for future earnings lost due to premature mortality
-
4.
Varying CVD prevalence by ± 10%
-
5.
Varying the proportions of people receiving non-statins after a non-fatal event by ± 15%
-
6.
Varying the prevalence and incidence of type 2 diabetes by ± 10%
-
7.
Varying 1-year CVD risk from the PCE equation by ± 15%
-
8.
Using an alternative disutility estimate from the Keng et al. study [60]
-
9.
Replacing moderate-intensity statins costs with high-intensity statins costs
-
10.
Replacing ezetimibe combined with a statin with PCSK9 inhibitor combined with a statin
2.10 Model Calibration and Validation
The model was calibrated using incidence rates of fatal and non-fatal cardiovascular events in Australia. The incidence rates of non-fatal MI and non-fatal stroke in people with type 2 diabetes were obtained from Morton et al. [12], and the incidence rates of CVD death for type 2 diabetes were obtained from the general Australian population based on 2021 ABS data [31]. Calibration ratios were then applied to the model.
The model was developed in Microsoft Excel and was examined for face validity to evaluate the appropriateness of the conceptual model, input data, and model outcomes through a series of consultation meetings with a panel of two experts in health economic modelling (ZA, CM). The model inputs were also varied to assess whether expected effects were predicted, and a manual review of formulae and a cross-check of all inputs were performed. Upon completion of the model examination, external validation was undertaken by using incidence and prevalence data of cardiovascular events and comparing the results of our model with those reported in national reports. The Assessment of the Validation Status of Health-Economic decision models (AdViSHE) tool was used to enhance the transparency of study model outcomes [61]. Sources of key model inputs with justifications are available in ESM Table 14.
3 Results
Between 2022 and 2031, a total of 1,153,078 and 166,612 individuals with type 2 diabetes were at risk of CVD and established CVD, respectively. In the first year, there were 497,332 males and 458,538 females with type 2 diabetes without prior CVD, aged 40–89 years, whereas by year 2031 there were 636,371 males and 516,707 females with type 2 diabetes without prior CVD. In the type 2 diabetes with established CVD population, there were 128,659 males and 58,094 females in 2022, while there were 100,207 males and 66,406 females in 2031.
3.1 Cardiovascular Events
Within a 10-year time horizon, the model projected 83,618 non-fatal MIs (95% uncertainty interval [UI] 83,170–84,053) and 58,774 non-fatal strokes (95% UI 58,458–59,013) would occur in Australians with type 2 diabetes aged 40–89 years old. The model also projected 50,204 deaths due to CVD causes (95% UI 50,143–50,273) and 262,039 deaths due to non-CVD causes (95% UI 256,109–266,767). Despite having the overall fatal and non-fatal CVD events, in both sexes, increase, fatal and non-fatal CVD events decreased in males with type 2 diabetes and established CVD (ESM Table 15).
3.2 Discounted Years of Life Lived, and QALYs
The projected total years of life lived in the population (with and without CVD) were 9,549,487 (95% UI 9,416,423–9,654,043), and the projected total years of life lived with CVD in the population (with and without CVD) were 1,411,574 (95% UI 1,266,736–1,542,298). Likewise, the total QALYs in the population (with and without CVD) were 6,632,897 (95% UI 5,065,606–7,591,679), and the total QALYs with CVD in the population (with and without CVD) were 966,859 (95% UI 513,834–1,159,365). Tables 1 and 2 (results of the PSA with 95% UI) and ESM Table 15 show the health outcome results.
3.3 Discounted Direct Healthcare and Indirect Costs
From the healthcare and societal perspectives, the total costs attributed to type 2 diabetes with CVD through 2031 will exceed AU$18.66 billion (95% UI 4.67–45.78 billion).
3.3.1 Direct Healthcare Costs
The total healthcare cost of type 2 diabetes (with and without established CVD) is projected to be AU$9.59 billion (95% UI 1.90–30.45 billion), with higher costs in males than in females, AU$5.80 versus AU$3.78 billion. The key drivers were the chronic costs of CVD, which contributed to 58.17% (AU$5.58 billion, 95% UI 145.22 million–24.816 billion) of total healthcare cost, followed by acute costs contributing to 21.83% (AU$2.09 billion, 95% UI 160.92 million–6.09 billion) of total healthcare cost, while pharmacological treatment contributed to 20% (AU$1.92 billion, 95% UI 33.49 million–10.57 billion) of total healthcare cost.
3.3.2 Indirect Costs
The total cost of productivity losses due to type 2 diabetes with and without CVD is projected to exceed AU$9.07 billion (95% UI 663.53 million–33.19 billion), with higher costs in males than in females, AU$4.93 versus 4.14 billion. The total cost was mainly driven by the productivity loss due to morbidity, which contributed to 57.35% of total productivity loss cost with AU$5.20 billion (95% UI 221.06 million–16.15 billion), followed by the productivity loss due to premature mortality, which contributed to 42.65% of total productivity loss cost with AU$3.87 billion (95% UI 213.39 million–20.65 billion). Tables 1 and 2 (results of PSA with 95% UI) and ESM Table 16 show the total economic outcomes for type 2 diabetes with and without established CVD.
ESM Tables 17 and 18 show the health and economic outcomes in the type 2 diabetes with and without CVD populations (undiscounted).
3.4 Sensitivity Analyses–Scenario Analyses
We found that changing the discount rate to 0% and varying the prevalence of CVD and type 2 diabetes by ±10% had the greatest influence on the overall outcomes. Varying the CVD prevalence by +10% increased the total healthcare and productivity loss costs to AU$10.07 billion and AU$9.55 billion, respectively, and varying the prevalence of type 2 diabetes by +10% increased the total healthcare and productivity loss costs to AU$10.12 billion and AU$9.52 billion, respectively. The remaining scenario analyses had minimal impact on the results (Table 3).
3.5 Model Calibration and Validation
External validation suggests that our model may overestimate the burden of CVD in type 2 diabetes in Australia. The AdViSHE checklist is presented in ESM Table 19. Our model was validated by comparing the model's findings versus the results reported by the AIHW and Morton et al. study [12, 39]. Additionally, our findings were compared against the results of the study by Marquina et al., which estimated the burden of CVD among Australians [21]. Our model over predicted non-fatal MI, non-fatal stroke, and CVD death in primary and secondary prevention. Calibration results are presented in ESM Table 20.
The CHEERS assessment is presented in ESM Table 21.
4 Discussion
This study is the first to report the health and economic impact of CVD among people with type 2 diabetes over the next decade and the first in the literature to use a multistate dynamic model, which reflected predicted changes in migration and death, as well as incident type 2 diabetes over time.
Our findings show that CVD in the type 2 diabetes population will substantially impact the healthcare system and society by 2031, in line with recent Australian reports [62]. Our projections indicate that the combined number of fatal and non-fatal CVD events experienced by Australians with type 2 diabetes aged 40–89 years will be 50,204 and 142,392, respectively, between 2022 and 2031.
The model also expected that the total costs among Australians with type 2 diabetes, with and without CVD, will exceed AU$18.66 billion from a societal perspective, with higher costs in males than in females, which might be due to more males having type 2 diabetes with and without CVD.
Furthermore, fatal and non-fatal CVD events decreased over time in males with type 2 diabetes with established CVD, which might be explained by lower life expectancy [63].
Our model demonstrated more QALYs and less total costs in people with type 2 diabetes without CVD compared to those with type 2 diabetes and CVD (5,730,796 vs 902,101) and (AU$7.67 billion vs AU$10.78 billion), respectively. Importantly, our findings showed that there will be a significant decrease in the total cost of type 2 diabetes and CVD by 2031 compared to 2022 (AU$834.67 million vs AU$1.46 billion). This is mostly driven by the decrease in the size of the Australian population with type 2 diabetes and CVD from 186,753 males and females in 2022 to 166,612 males and females by 2031. It could also be due to the increased mortality events (34,924 in 2031 vs 27,040 in 2022). This is in line with a recent study in Australia that showed that mortality due to diabetes increased from 2010 to 2016, with an average of nearly 3% annually [64].
Results of previous studies are not comparable to our findings due to expected different approaches to modelling. Previous studies were based on the simple Markov cohort, versus a multistate dynamic model in our study. The multistate dynamic nature of our model allowed for demographic variations over time and incidence of type 2 diabetes to reflect a more realistic picture of CVD burden and projection of CVD burden in type 2 diabetes in Australia. Additionally, while in fixed Markov models results are reported per person, it is a challenge to include per person outcomes in dynamic models. Previous published dynamic models [21, 65,66,67,68,69] have only reported the total impact, with an explanation that every individual in the model has a different follow-up, and this will not represent true costs per person over 10 years.
A systematic review by Einarson et al. [70] included data from 13 countries ranging from less than 1 year to 10 years of follow-up, and revealed that CVD costs contributed between 20 and 49% of the total healthcare cost of managing type 2 diabetes. The total costs comprised inpatient and outpatient costs (i.e., emergency department visits, hospitalization, pharmaceuticals, laboratory, and physician visits). The same study reported excess costs of CVD of US$3418–US$9705 per person per year as opposed to those without CVD in the type 2 diabetes population. Furthermore, Einarson et al.’s study [70] found that the healthcare cost of managing CVD due to type 2 diabetes was mostly attributed to hospital’s resource utilization compared to chronic costs, which was the key driver in our study. Studies included in this systematic review [70] were capturing only direct cost of disease; this in return may largely underestimate the actual economic burden of CVD in type 2 diabetes. Another systematic review, by Vaidya et al. [71], included studies from six countries and revealed that CVD (MI/stroke) was the second contributor to the healthcare burden among people with type 2 diabetes after end-stage renal disease. The total healthcare costs (i.e., emergency visits, hospitalization, outpatient costs, and prescription medication) ranged from US$8805 to US$9648 per person with type 2 diabetes and CVD in the US. However, the Vaidya et al. study [71] focused on the prevalence of CVD complications in people with type 2 diabetes rather than new incidence cases.
A study by Straka et al. [72] reported that people with type 2 diabetes incurred higher direct medical costs for cardiovascular care during their first hospitalization than those without type 2 diabetes in the US. The total healthcare costs in people with type 2 diabetes and CVD over a 3-year follow-up period in Straka et al. was US$16,149 per person.
In Australia, a previous study by Marquina et al. portrayed the burden of CVD among general Australians irrespective of their diabetes status [21]. Although this study demonstrates the total burden of CVD in Australians, less than 10% of the Australians included in the model comprised people with type 2 diabetes. Thus, the study did not reflect the true picture of the health and economic impact of type 2 diabetes. The Marquina et al. study reported 377,754 fatal cardiovascular events and 991,375 non-fatal cardiovascular events, compared to 50,204 fatal cardiovascular events and 142,392 non-fatal cardiovascular events, respectively, in our current study. The reported total costs in the Marquina et al. study were also higher than in the current study, AU$140.65 billion versus AU$18.66 billion. This is likely reflecting the higher number of Australians with and without CVD in the Marquina et al. study compared to only the type 2 diabetes population in the current study [21]. Of note, Marquina et al. did not capture the incidence of type 2 diabetes in those initially without type 2 diabetes. This is essential to designing strategies focused on CVD complications associated with management of type 2 diabetes.
Our study, as such will serve as a foundation for future work to investigate different strategies to optimize the control of different risk factors for the prevention and treatment of CVD in Australians with type 2 diabetes using comprehensive dynamic models. The dynamic nature of our model might also help inform the budget impact analysis of future treatments or prevention programs. It is crucial to allocate resources efficiently, design strategies focused on type 2 diabetes patients, and aid policy makers and clinicians in prioritizing interventions to prevent and manage CVD in type 2 diabetes.
We acknowledge several limitations in our model. Firstly, clinicians have expressed concerns in relation to the 2013 PCE-ASCVD algorithm performance for the type 2 diabetes without CVD population, as it has been only validated among adults aged 40–79 years, and suboptimal calibration in people 80 years or older may overestimate the risk [73]. However, the 2013 PCE-ASCVD has been validated, and demonstrated better performance, in Australians with type 2 diabetes, compared to 1991 Framingham, 2008 Framingham, and 2008 office-based Framingham [34] models, and has been recommended in clinical practice for risk stratification to guide primary prevention strategies for people with ASCVD [32]. Furthermore, the current study calibration results revealed that the PCE-ASCVD performed better against Australian mortality and incidence rates data. We did not use the Fremantle diabetes-specific CVD risk equation for Australians, as it has been validated to predict 5-year CVD risk and was based on a population from Western Australia only [74]. Additionally, the Fremantle risk equation did not include smoking, hypertension treatment, or systolic blood pressure as risk factors. In contrast, the 2013 PCE-ASCVD validation was based on the AusDiab study population, a national, longitudinal, population-based cohort study in six Australian states and the Northern Territory, and followed people for 10 years [34].
A second limitation is that we used data from the 2011–2012 NHS, where only 313 Australians with type 2 diabetes, aged 40–90 years were available. However, the NHS data represents the only nationally available sample population in Australia for these purposes. Another limitation is that we did not consider the equity issues in the model, such as socioeconomic characteristics. Based on a recent study, CVD is expected to influence Australians in the lowest socioeconomic quintile the most [66]. Also a limitation, with regard to the type 2 diabetes with established CVD population, is that we obtained the CVD risk from the REACH registry [38], which is limited to 4 years follow-up only, but the REACH registry is a global registry where almost 3000 people were enrolled from Australia.
An added limitation is that the proportions of fatal and non-fatal MI and stroke events among Australians were not stratified according to age and sex. This may result in an overestimation or underestimation of the effects. In addition, it was difficult to account for employee replacement costs in the model. However, our model may more accurately reflect the real-world picture of lost productivity, in which replacement of employees may not always be possible [75]. In any case, including this information will further increase the productivity loss (i.e., overestimate the effect). A seventh limitation is that the model also did not consider the on-costs for employees, such as payroll tax and compensation insurance, as these were difficult to accurately estimate, which may overestimate the productivity loss (i.e., overestimate the burden). The fact that the model omits intangible costs and costs associated with traveling time and spillover effects on families due to disease burden is another model limitation, which may underestimate the indirect costs. It is also important to acknowledge that other lost productivity costs of complications that are associated with type 2 diabetes, such as retinopathy, nephropathy, and neuropathy, were not accounted for in the model. Including this information, however, will only increase the economic burden of productivity loss. Finally, the performance of our projection model might lead to different findings depending on the input variables. For example, when we considered multiple scenario analyses to account for optimal and suboptimal risk-factor control, such as varying type 2 diabetes and CVD prevalence by ± 10%, the number of fatal and non-fatal CVD events and overall costs changed.
5 Conclusion
In conclusion, our analysis provides original insights and projects nationally representative estimates of CVD’s societal burden among people with type 2 diabetes in Australia between 2022 and 2031.
References
De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Front Endocrinol (Lausanne). 2018;9:2.
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83.
Indicators for the Australian National Diabetes Strategy 2016–2020: data update 09 December 2020. https://www.aihw.gov.au/reports/diabetes/diabetes-indicators-strategy-2016-2020/contents/goal-3-reduce-the-occurrence-of-diabetes-related-complications-improve-quality-of-life/indicator-3-7-prevalence-of-cardiovascular-disease-among-people-with-diabetes. Accessed 15 May 2022.
Baker IDI Heart and Diabetes Institute. Diabetes: the silent pandemic and its impact on Australia. Melbourne: Baker IDI Heart and Diabetes Institute. 2012. https://www.baker.edu.au/-/media/Documents/impact/diabetes-the-silent-pandemic.ashx?la=en. Accessed 5 Mar 2022.
Taylor KS, Heneghan CJ, Farmer AJ, Fuller AM, Adler AI, Aronson JK, et al. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large UK primary care database. Diabetes Care. 2013;36(8):2366–71.
Jansson SPO, Andersson DKG, Svärdsudd K. Mortality trends in subjects with and without diabetes during 33 years of follow-up. Diabetes Care. 2010;33(3):551–6.
Dale AC, Vatten LJ, Nilsen TI, Midthjell K, Wiseth R. Secular decline in mortality from coronary heart disease in adults with diabetes mellitus: cohort study. BMJ. 2008;337(7661): a236.
Tancredi M, Rosengren A, Svensson A-M, Kosiborod M, Pivodic A, Gudbjörnsdottir S, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–32.
Lind M, Garcia-Rodriguez LA, Booth GL, Cea-Soriano L, Shah BR, Ekeroth G, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia. 2013;56(12):2601–8.
Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet (London, England). 2018;391(10138):2430–40.
Harding JL, Shaw JE, Peeters A, Guiver T, Davidson S, Magliano DJ. Mortality trends among people with type 1 and type 2 diabetes in Australia: 1997–2010. Diabetes Care. 2014;37(9):2579–86.
Morton JI, Lazzarini PA, Shaw JE, Magliano DJ. Trends in the incidence of hospitalization for major diabetes-related complications in people with type 1 and type 2 diabetes in Australia, 2010–2019. Diabetes Care. 2022;45(4):789–97.
Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725–31.
Laiteerapong N, Huang ES, Chin MH. Prioritization of care in adults with diabetes and comorbidity. Ann NY Acad Sci. 2011;1243:69–87.
Kellow N, Khalil H. A review of the pharmacological management of type 2 diabetes in a rural Australian primary care cohort. Int J Pharm Pract. 2013;21(5):297–304.
Chalasani S, Peiris DP, Usherwood T, Redfern J, Neal BC, Sullivan DR, et al. Reducing cardiovascular disease risk in diabetes: a randomised controlled trial of a quality improvement initiative. Med J Aust. 2017;206(10):436–41.
Simons LA, Chung E. Are high coronary risk patients missing out on lipid-lowering drugs in Australia? Med J Aust. 2014;201(4):213–6.
Marson A, Raffoul N, Osman R, Deed G. Management of patients with type 2 diabetes and cardiovascular disease in primary care. Aust J Gen Pract. 2021;50(4):238–45.
Lee CMY, Goode B, Nørtoft E, Shaw JE, Magliano DJ, Colagiuri S. The cost of diabetes and obesity in Australia. J Med Econ. 2018;21(10):1001–5.
Diabetes: Australian facts. 2022. https://www.aihw.gov.au/reports/diabetes/diabetes-australian-facts/contents/impact-of-diabetes/health-system-expenditure. Accessed 15 May 2022.
Marquina C, Talic S, Vargas-Torres S, Petrova M, Abushanab D, Owen A, et al. Future burden of cardiovascular disease in Australia: impact on health and economic outcomes between 2020 and 2029. Eur J Prev Cardiol. 2022;29(8):1212–9.
3101.0—Australian Demographic Statistics, Jun 2019. https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Jun2019?OpenDocument. Accessed 22 Jan 2022.
Backholer K, Hirakawa Y, Tonkin A, Giles G, Magliano DJ, Colagiuri S, et al. Development of an Australian cardiovascular disease mortality risk score using multiple imputation and recalibration from national statistics. BMC Cardiovasc Disord. 2017;17(1):17.
Australia Life Expectancy 1950–2022. https://www.macrotrends.net/countries/AUS/australia/life-expectancy#:~:text=Thecurrentlifeexpectancyfor,a0.18%25increasefrom2019. Accessed 11 Jan 2022.
Abushanab D, Liew D, Marquina C, Al-Badriyeh D, Ademi Z. Cost-effectiveness of empagliflozin and metformin combination versus standard care as first-line therapy in patients with type 2 diabetes mellitus. Endocr Pract. 2022;28(1):16–24.
Abushanab D, Al-Badriyeh D, Liew D, Ademi Z. First-line treatment with empagliflozin and metformin combination versus standard care for patients with type 2 diabetes mellitus and cardiovascular disease in Qatar. A cost-effectiveness analysis. Curr Probl Cardiol. 2022;47(6): 100852.
National Health & Medical Research Council. How to compare the costs and benefits: evaluation of the economic evidence. https://www.nhmrc.gov.au/sites/default/files/images/how-to-compare-costs-and-benefits-evaluation-of-the-economic-evidence.pdf. Accessed 20 May 2022.
Health technology assessments 2022. https://www.health.gov.au/health-topics/health-technologies-and-digital-health/health-technology-assessments. Accessed 20 May 2022.
Husereau D, Drummond M, Augustovski F, Briggs AH, Carswell C, Caulley L, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Pharmacoeconomics. 2022;40:601–9.
Australian Institute of Health and Welfare. https://www.aihw.gov.au/. Accessed 13 Mar 2022.
Heart, stroke and vascular disease—Australian facts 2022. https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/hsvd-facts/contents/heart-stroke-and-vascular-disease-and-subtypes/coronary-heart-disease. Accessed 22 Jan 2022.
Goff DCJ, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S49-73.
Australian Bureau of Statistics. 4364.0.55.001—National Health Survey: first results, 2017–18. https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/4364.0.55.0012017-18?OpenDocument. Accessed 13 Mar 2022.
Albarqouni L, Doust JA, Magliano D, Barr EL, Shaw JE, Glasziou PP. External validation and comparison of four cardiovascular risk prediction models with data from the Australian Diabetes, Obesity and Lifestyle study. Med J Aust. 2019;210(4):161–7.
No Postcode Untouched Stroke in Australia. 2020. https://strokefoundation.org.au/What-we-do/Research/No-Postcode-Untouched. Accessed 13 Mar 2022.
Coronary heart disease. 2020. https://www.aihw.gov.au/reports/australias-health/coronary-heart-disease. Accessed 13 Mar 2022.
Population Projections, Australia. 2018. https://www.abs.gov.au/statistics/people/population/population-projections-australia/latest-release. Accessed 13 Mar 2022.
Krempf M, Parhofer KG, Steg PG, Bhatt DL, Ohman EM, Röther J, et al. Cardiovascular event rates in diabetic and nondiabetic individuals with and without established atherothrombosis (from the REduction of Atherothrombosis for Continued Health [REACH] Registry). Am J Cardiol. 2010;105(5):667–71.
Australian Government. Australian Institute of Health and Welfare. General Record of Incidence of Mortality (GRIM) data. https://www.aihw.gov.au/reports/life-expectancy-deaths/grim-books/contents/general-record-of-incidence-of-mortality-grim-data. Accessed 14 May 2022.
Carstensen B, Rønn PF, Jørgensen ME. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996–2016. BMJ Open Diabetes Res Care. 2020;8(1): e001071.
McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. 2016;14(1):133.
Glasziou P, Alexander J, Beller E, Clarke P, Group AC. Which health-related quality of life score? A comparison of alternative utility measures in patients with type 2 diabetes in the ADVANCE trial. Health Qual Life Outcomes. 2007;5:21.
The Royal Australian College of General Practitioners. General practice management of type 2 diabetes 2020. https://www.racgp.org.au/getattachment/41fee8dc-7f97-4f87-9d90-b7af337af778/Management-of-type-2-diabetes-A-handbook-fo. Accessed 11 Dec 2022.
Brett T, Radford J, Qureshi N, Pang J, Watts GF. Evolving worldwide approaches to lipid management and implications for Australian general practice. Aust J Gen Pract. 2021;50(5):297–304.
Australian Government. Department of Health. Pharmaceutical Benefits Scheme (PBS). http://www.pbs.gov.au/pbs/home;jsessionid=11b0snpn6tl8g180rspms6zb3. Accessed 22 Mar 2022.
Morton JI, Ilomӓki J, Magliano DJ, Shaw JE. Persistent disparities in diabetes medication receipt by socio-economic disadvantage in Australia. Diabet Med. 2022;39(9): e14898.
Talic S, Marquina C, Zomer E, Ofori-Asenso R, Petrova M, Vargas-Torres S, et al. Attainment of low-density lipoprotein cholesterol goals in statin treated patients: real-world evidence from Australia. Curr Probl Cardiol. 2022;47(7): 101068.
Australian refined diagnosis-related groups (AR-DRG) data cubes. https://www.aihw.gov.au/reports/hospitals/ar-drg-data-cubes/contents/data-cubes. Accessed 3 Mar 2022.
Clarke P, Leal J, Kelman C, Smith M, Colagiuri S. Estimating the cost of complications of diabetes in Australia using administrative health-care data. Value Health J Int Soc Pharmacoecon Outcomes Res. 2008;11(2):199–206.
Deaths in Australian hospitals 2014–15. https://www.aihw.gov.au/reports/hospitals/deaths-in-australian-hospitals-2014-15/contents/summary/how-many-deaths-occur-in-hospital. Accessed 25 June 2022.
Pike J, Grosse SD. Friction cost estimates of productivity costs in cost-of-illness studies in comparison with Human Capital Estimates: a review. Appl Health Econ Health Policy. 2018;16(6):765–78.
Vyas MV, Hackam DG, Silver FL, Laporte A, Kapral MK. Lost productivity in stroke survivors: an econometrics analysis. Neuroepidemiology. 2016;47(3–4):164–70.
Worcester MU, Elliott PC, Turner A, Pereira JJ, Murphy BM, Le Grande MR, et al. Resumption of work after acute coronary syndrome or coronary artery bypass graft surgery. Heart Lung Circ. 2014;23(5):444–53.
Australian Bureau of Statistics. Average weekly earnings, Australia. 2021. https://www.abs.gov.au/ausstats/abs@.nsf/mf/6302.0. Accessed 22 Mar 2022.
Labour Force, Australia .2021. https://www.abs.gov.au/ausstats/abs@.nsf/mf/6202.0. Accessed 22 Mar 2022.
Services Australia We deliver government payments and services. 2021. Available from: https://www.servicesaustralia.gov.au/. Accessed 9 May 2022.
Passey ME, Shrestha RN, Bertram MY, Schofield DJ, Vos T, Callander EJ, et al. The impact of diabetes prevention on labour force participation and income of older Australians: an economic study. BMC Public Health. 2012;12:16.
Westerlind E, Persson HC, Sunnerhagen KS. Return to work after a stroke in working age persons. A six-year follow up. PLoS ONE. 2017;12(1):e0169759–e0169759.
Australian Institute of Health and Welfare 2019. Health Expenditure Australia 2017–18. Australian Government; 2019. (Health and welfare expenditure series no. 65). Report No.: Cat. no. HWE 77. https://www.aihw.gov.au/. Accessed 3 Mar 2022.
Keng MJ, Leal J, Bowman L, Armitage J, Mihaylova B. Decrements in health-related quality of life associated with adverse events in people with diabetes. Diabetes Obes Metab. 2022;24(3):530–8.
Vemer P, Corro Ramos I, van Voorn GAK, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61.
Australian Burden of Disease Study 2018—key findings. 2018. https://www.aihw.gov.au/reports/burden-of-disease/burden-of-disease-study-2018-key-findings/contents/key-findings. Accessed 29 May 2022.
Life expectancy hits a new high. 2021. https://www.abs.gov.au/media-centre/media-releases/life-expectancy-hits-new-high. Accessed 29 May 2022.
Wijnen A, Bishop K, Joshy G, Zhang Y, Banks E, Paige E. Observed and predicted premature mortality in Australia due to non-communicable diseases: a population-based study examining progress towards the WHO 25X25 goal. BMC Med. 2022;20(1):57.
Savira F, Ademi Z, Wang BH, Kompa AR, Owen AJ, Liew D, et al. The preventable productivity burden of kidney disease in Australia. J Am Soc Nephrol. 2021;32(4):938–49.
Hastings K, Marquina C, Morton J, Abushanab D, Berkovic D, Talic S, et al. Projected new-onset cardiovascular disease by socioeconomic group in Australia. Pharmacoeconomics. 2022;40(4):449–60.
Menon K, de Courten B, Ademi Z, Owen AJ, Liew D, Zomer E. Estimating the benefits of obesity prevention on productivity: an Australian perspective. Int J Obes. 2022;46(8):1463–9.
Menon K, de Courten B, Liew D, Ademi Z, Owen AJ, Magliano DJ, et al. Productivity benefits of preventing type 2 diabetes in Australia: a 10-year analysis. Diabetes Care. 2021;44(3):715–21.
Savira F, Wang BH, Kompa AR, Ademi Z, Owen AJ, Liew D, et al. The impact of coronary heart disease prevention on work productivity: a 10-year analysis. Eur J Prev Cardiol. 2021;28(4):418–25.
Einarson TR, Acs A, Ludwig C, Panton UH. Economic burden of cardiovascular disease in type 2 diabetes: a systematic review. Value Health. 2018;21(7):881–90.
Vaidya V, Gangan N, Sheehan J. Impact of cardiovascular complications among patients with type 2 diabetes mellitus: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2015;15(3):487–97.
Straka RJ, Liu LZ, Girase PS, DeLorenzo A, Chapman RH. Incremental cardiovascular costs and resource use associated with diabetes: an assessment of 29,863 patients in the US managed-care setting. Cardiovasc Diabetol. 2009;8:53.
Cook NR, Ridker PM. Calibration of the pooled cohort equations for atherosclerotic cardiovascular disease: an update. Ann Intern Med. 2016;165(11):786–94.
Davis WA, Knuiman MW, Davis TME. An Australian cardiovascular risk equation for type 2 diabetes: the Fremantle Diabetes Study. Intern Med J. 2010;40(4):286–92.
Lensberg BR, Drummond MF, Danchenko N, Despiégel N, François C. Challenges in measuring and valuing productivity costs, and their relevance in mood disorders. Clinicoecon Outcomes Res. 2013;5:565–73.
Acknowledgements
No potential conflicts of interest relevant to this article were reported. The authors would like to thank the two anonymous reviewers for their review of the manuscript and insightful suggestions.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors have no conflicts of interest to disclose.
Availability of data and material
All data analyzed during this work are included in this published article and its ESM file, and in the relevant references. Any additional data are available on request from authors.
Code availability
The model was performed in @Risk-7.5® (Palisade Corporation, NY, USA), and is available on request from authors.
Ethics approval
The study did not require ethics approval.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
DA was involved in data acquisition, development of the model, data analysis and interpretation, and wrote the first draft of the manuscript. CM was involved in the design, data acquisition, and analysis. JM was involved in data acquisition and data interpretation. DL and DAB contributed to analysis and interpretation of data. ML and DM contributed to interpretation of data. ZA led the study’s conception and design and contributed to data analysis and interpretation and the written manuscript. All authors contributed to revising and approving the manuscript. DA and ZA are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Abushanab, D., Marquina, C., Morton, J.I. et al. Projecting the Health and Economic Burden of Cardiovascular Disease Among People with Type 2 Diabetes, 2022–2031. PharmacoEconomics 41, 719–732 (2023). https://doi.org/10.1007/s40273-023-01258-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01258-7




 : Markov.
: Markov.  : end cycle
: end cycle