Abstract
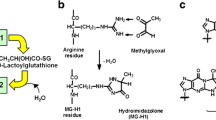
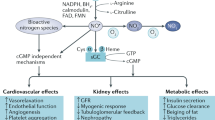
Increased glycolytic flux into the diabetic kidney, combined with glycolytic inefficiencies introduced by oxidative stress, acts to increase the generation of triose-phosphate intermediates, which spontaneously degrade to form methylglyoxal. At the same time, the glyoxalase-catalysed pathway that degrades excess methylglyoxal is impaired. The resulting dicarbonyl stress increases the accumulation of Advanced Glycation End-products (AGEs), as highly reactive dicarbonyls modify proteins, DNA, phospholipids and even small molecules like glutathione and nitric oxide. The resulting molecular dysfunction, contributes to the development and progression of kidney disease in diabetes. The importance of the dicarbonyls in diabetic kidney disease is clearly demonstrated by the reno-protective benefits of structurally-disparate dicarbonyl scavengers in experimental studies. Equally, modulating the glyoxalase pathway is able to alter both dicarbonyl generation and renal dysfunction in the presence and absence of hyperglycaemia. However, beyond improving glycemia control and reducing oxidative stress, an effective way to attenuate dicarbonyl-mediated damage in patients with diabetic kidney disease remains an elusive goal.


Similar content being viewed by others
References
Fleming T, Cuny J, Nawroth G et al (2012) Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates? Diabetologia 55(4):1151–1155
Golej J, Hoeger H, Radner W, Unfried G, Lubec G (1998) Oral administration of methylglyoxal leads to kidney collagen accumulation in the mouse. Life Sci 63(9):801–807
Rodrigues L, Matafome P, Crisostomo J et al (2014) Advanced glycation end products and diabetic nephropathy: a comparative study using diabetic and normal rats with methylglyoxal-induced glycation. J Physiol Biochem 70(1):173–184
Kim J, Sohn E, Kim CS, Kim JS (2011) Renal podocyte apoptosis in Zucker diabetic fatty rats: involvement of methylglyoxal-induced oxidative DNA damage. J Comp Pathol 144(1):41–47
Giacco F, Du X, D'Agati VD et al (2014) Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 63(1):291–299
Brouwers O, Niessen PMG, Miyata T et al (2014) Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 57(1):224–235
Thornalley PJ (1996) Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification—a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmac 27(4):565–573
Thornalley PJ (2003) Glyoxalase I-structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 31(6):1343
Thomas MC (2011) Advanced glycation end products. Contrib Nephrol 170:66–74
Toxicity Co. Statement on methylglyoxal. UK Government; 2018. TOX/2009/38
Dhar A, Desai K, Kazachmov M, Yu P, Wu L (2008) Methylglyoxal production in vascular smooth muscle cells from different metabolic precursors. Metabolism 57(9):1211–1220
Du X, Matsumura T, Edelstein D et al (2003) Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112(7):1049–1057
Hanssen NMJ, Scheijen J, Jorsal A et al (2017) Higher plasma methylglyoxal levels are associated with incident cardiovascular disease in individuals with type 1 diabetes: a 12-year follow-up study. Diabetes 66(8):2278–2283
Bierhaus A, Fleming T, Stoyanov S et al (2012) Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med 18(6):926–933
Thornalley PJ (2005) Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci 1043:111–117
Thornalley PJ (2008) Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems–role in ageing and disease. Drug Metabol Drug Interact 23(1–2):125–150
Pillin A, Pudil F, Bencko V, Bezdickova D (2007) Contents of pentosidine in the tissue of the intervertebral disc as an indicator of the human age. Soud Lek 52(4):60–64
Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycation end-products: a review. Diabetologia 44(2):129–146
Thomas MC, Tikellis C, Burns WM et al (2005) Interactions between renin angiotensin system and advanced glycation in the kidney. J Am Soc Nephrol 16(10):2976–2984
Rosca MG, Mustata TG, Kinter MT et al (2005) Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am J Physiol Renal Physiol 289(2):F420–430
Bulteau AL, Verbeke P, Petropoulos I, Chaffotte AF, Friguet B (2001) Proteasome inhibition in glyoxal-treated fibroblasts and resistance of glycated glucose-6-phosphate dehydrogenase to 20 S proteasome degradation in vitro. J Biol Chem 276(49):45662–45668
Queisser MA, Yao D, Geisler S et al (2010) Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 59(3):670–678
Aldini G, Vistoli G, Stefek M et al (2013) Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radical Res 47(sup1):93–137
Fessel G, Li Y, Diederich V et al (2014) Advanced glycation end-products reduce collagen molecular sliding to affect collagen fibril damage mechanisms but not stiffness. PLoS ONE 9(11):e110948–e110948
Rabbani N, Thornalley PJ (2014) Dicarbonyl proteome and genome damage in metabolic and vascular disease. Biochem Soc Trans 42(2):425–432
Galligan JJ, Wepy JA, Streeter MD et al (2018) Methylglyoxal-derived posttranslational arginine modifications are abundant histone marks. Proc Natl Acad Sci 115(37):9228
Gugliucci A, Bendayan M (1995) Histones from diabetic rats contain increased levels of advanced glycation end products. Biochem Biophys Res Commun 212(1):56–62
El-Osta A, Brasacchio D, Yao D et al (2008) Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205(10):2409–2417
Bucala R, Tracey KJ, Cerami A (1991) Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest 87(2):432–438
Deuther-Conrad W, Franke S, Sommer M, Henle T, Stein G (2001) Differences in the modulating potential of advanced glycation end product (AGE) peptides versus AGE proteins. Kidney Int Suppl 78:S63–66
Yamamoto Y, Kato I, Doi T et al (2001) Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest 108(2):261–268
Watson AM, Gray SP, Jiaze L et al (2012) Alagebrium reduces glomerular fibrogenesis and inflammation beyond preventing RAGE activation in diabetic apolipoprotein E knockout mice. Diabetes 61(8):2105–2113
Pickering RJ, Tikellis C, Rosado CJ et al (2019) Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J Clin Invest 129(1):406–421
Watanabe M, Toyomura T, Wake H, et al. Differential contribution of possible pattern-recognition receptors to advanced glycation end product-induced cellular responses in macrophage-like RAW264.7 cells. Biotechnol Appl Biochem. 2019.
Hanssen NMJ, Stehouwer CDA, Schalkwijk CG (2019) Methylglyoxal stress, the glyoxalase system, and diabetic chronic kidney disease. Curr Opin Nephrol Hypertens 28(1):26–33
Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M (2013) Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care 36(10):3234–3239
Lu J, Randell E, Han Y, Adeli K, Krahn J, Meng QH (2011) Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin Biochem 44(4):307–311
Ito K, Sakata N, Nagai R et al (2017) High serum level of methylglyoxal-derived AGE, Ndelta-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine, independently relates to renal dysfunction. Clin Exp Nephrol 21(3):398–406
Thomas MC, Woodward M, Neal B et al (2015) Relationship between levels of advanced glycation end products and their soluble receptor and adverse outcomes in adults with type 2 diabetes. Diabetes Care 38(10):1891–1897
Genuth S, Sun W, Cleary P et al (2015) Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes 64(1):266–278
Velayoudom-Cephise FL, Rajaobelina K, Helmer C et al (2016) Skin autofluorescence predicts cardio-renal outcome in type 1 diabetes: a longitudinal study. Cardiovasc Diabetol 15(1):127
Schumacher D, Morgenstern J, Oguchi Y et al (2018) Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol Metab 18:143–152
Yamazoye S (1936) GLYOXALASE AND ITS CO-ENZYME: III. The mechanism of the action of glutathione as the co-enzyme of glyoxalase. J Biochem 23(2):319–334
Rabbani N, Xue M, Thornalley PJ (2016) Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj J 33(4):513–525
Morgenstern J, Fleming T, Schumacher D et al (2017) Loss of glyoxalase 1 induces compensatory mechanism to achieve dicarbonyl detoxification in mammalian schwann cells. J Biol Chem 292(8):3224–3238
Bartling B, Zunkel K, Al-Robaiy S, Dehghani F, Simm A (2019) Gene doubling increases glyoxalase 1 expression in RAGE knockout mice. Biochim Biophys Acta Gen Subj 129:438
Xue M, Rabbani N, Momiji H et al (2012) Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem J 443(1):213–222
Antognelli C, Trapani E, Delle Monache S et al (2018) KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: implication for cerebral cavernous malformation disease. Free Radical Biol Med 115:202–218
Xue M, Weickert MO, Qureshi S et al (2016) Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes 65(8):2282–2294
Qi W, Keenan HA, Li Q et al (2017) Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 23(6):753–762
Shafie A, Xue M, Barker G, Zehnder D, Thornalley PJ, Rabbani N (2016) Reappraisal of putative glyoxalase 1-deficient mouse and dicarbonyl stress on embryonic stem cells in vitro. Biochem J 473(22):4255–4270
Bolton WK, Cattran DC, Williams ME et al (2004) Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol 24(1):32–40
Williams ME, Bolton WK, Khalifah RG, Degenhardt TP, Schotzinger RJ, McGill JB (2007) Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am J Nephrol 27(6):605–614
Davies SS, Zhang LS (2017) Reactive carbonyl species scavengers-novel therapeutic approaches for chronic diseases. Curr Pharmacol Rep 3(2):51–67
Zhang F, Masania J, Anwar A et al (2016) The uremic toxin oxythiamine causes functional thiamine deficiency in end-stage renal disease by inhibiting transketolase activity. Kidney Int 90(2):396–403
Alkhalaf A, Klooster A, van Oeveren W et al (2010) A double-blind, randomized, placebo-controlled clinical trial on benfotiamine treatment in patients with diabetic nephropathy. Diabetes Care 33(7):1598–1601
Acknowledgements
MCT is supported by an NHMRC Senior Research Fellowship. AD is a recipient of an Australian Government RTP stipend.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MCT and CJR have filed patents pertaining to the Receptor of Advanced Glycation End-products (RAGE). AD has no conflict of interest.
Ethical approval
This article is a review of the literature, and as such, its compilation or submission did not require formal informed consent from human participants and/or animals, or ethical clearance according to standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dimitropoulos, A., Rosado, C.J. & Thomas, M.C. Dicarbonyl-mediated AGEing and diabetic kidney disease. J Nephrol 33, 909–915 (2020). https://doi.org/10.1007/s40620-020-00718-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00718-z