Abstract
Introduction
To determine the cost of depression comorbidity among Japanese adults with rheumatoid arthritis (RA).
Methods
A retrospective database study of 8968 patients diagnosed with RA between 2010 and 2015 and treated with any RA medication was conducted. Health care utilization characteristics were compared between patients with and without a comorbidity of depression. Propensity score matching was applied to ensure a balanced comparison between the two cohorts.
Results
The prevalence of a depression comorbidity was found for 5% of the total RA patients. This comorbidity was associated with 62% (56%) higher total outpatient visits and 66% (163%) higher rate of emergency room visits after 6 (12) months.
Conclusions
Burden of depression among RA patients in Japan is relatively high and awareness for depression as a comorbidity of RA needs to be reinforced.
Funding
Janssen Pharmaceutical KK.
Similar content being viewed by others
Introduction
Accumulating evidence from clinical and epidemiological studies indicates that depression is highly prevalent as a common comorbidity in patients affected with rheumatoid arthritis (RA) [1,2,3]. While the prevalence of depression differs significantly among studies, as high as 42% of patients with RA have been reported to be affected by depression [4]. Recently, a meta-analysis of 72 studies involving 13,189 individuals affected with RA found that 14.8–38.8% of such populations are often diagnosed with a major depressive disorder [5]. Furthermore, depression comorbidity of RA has been shown to be linked to a higher rate of unemployment, loss of productivity at work, as well as burden of escalating healthcare costs on both the affected individuals and society at large [6,7,8].
Frequently, depression comorbidity of RA remains undiagnosed and consequently under-treated [9], often due to the lack of communication about depression between the specialist and their patients [7, 10]. Previous studies have further found that individuals affected by depression comorbidity of RA are less likely to adhere to their medication regimens with subsequent elevated health care costs [7, 11]. For Japan, little is known about data on the health care utilization of depression comorbidity among RA patients. Hence, the purpose of the present study was to determine characteristics of health care utilization prevalent among the Japanese population affected with RA and comorbidity of depression.
Methods
Patient Population and Data Collection
We utilized the Japan Medical Data Center (JMDC) claims database from January 2010 to December 2015. The database includes health insurance claims data from non-governmental employees together with their family members. It also provides information on patient demographics, diagnostic codes, dates and types of procedures, dispensed prescription drugs, medical services available to inpatients and outpatients, as well as direct costs and expenditures. The JMDC database has been used to investigate a wide range of conditions in Japan such as schizophrenia, diabetes, and cardiovascular disease [12, 13]. To protect patient confidentiality, all personally identifiable information was de-identified, and as such no informed consent was deemed necessary.
We included RA patients with at least two ICD 10th diagnoses of RA (M05, M06.0, M06.2-M06.9). Moreover, patients were required to have received at least two prescriptions for RA treatment [DMARDs/Biologic]. Patients less than 18 years of age and affected with Crohn’s disease, ankylosing spondylitis, juvenile arthritis, psoriasis, ulcerative colitis, psoriatic arthritis, and/or Behçet’s disease were excluded from the analysis. Depression as a comorbidity was defined when a patient had at least two ICD 10th diagnoses of depression such as major depressive disorder, dysthymic disorder, depressive disorder NOS, and depressive mood (F03, F32.0, F32.1, F32.2, F32.8, F32.9, F33.1, F33.2, F33.3, F33.9, F34.1, F34.9, F41.2, and F53.0). In addition, patients were required to have received at least two prescriptions for treatment of depression including selective serotonin reuptake inhibitors (SSRI), serotonin noradrenaline reuptake inhibitor (SNRI), tricyclic antidepressant (TCA), monoamine oxidase inhibitors (MAOI).
Statistical Analysis
Since there is a potential imbalance in baseline covariates between patients with and without depression, we performed a propensity score matching to eliminate the impact of confounding parameters. This procedure allows us to estimate resource and costs attributable to depression in patients with RA. Each patient of the reference cohort was matched with up to four comparison cases with the closest propensity score using a greedy algorithm without replacement (i.e., once a match is made, the match is not reconsidered). The maximum tolerated difference between matched subjects in a “non-perfect” matching (i.e., caliper matching) was 0.2 × standard deviation of the logit of the propensity score [14].
The propensity score was assessed using a multivariable logistic regression model. Each potential covariate of interest was first tested in a univariate model and retained for the multivariate logistic regression model if P < 0.10. The potential confounders include age at index date, gender, calendar year of index-date, comorbidity measures (i.e., Charlson comorbidity index within the pre-index period, renal failure, interstitial pneumonia, COPD, peptic ulcer, chronic liver disease, osteoporosis, diabetes), polypharmacy in the pre-index period, and follow-up duration. The Charlson comorbidity index (CCI) is used to measure 19 comorbidities by assigning a weight between 1 and 6 to each one with higher CCI indicative of greater morbidity affecting the patient [15].
The Kaplan–Meier survival plots were used to estimate the proportions of patients with at least one occurrence of health care utilization (HCU), while the difference between the two groups (RA/depression and RA only) was assessed by the log-rank test. An adjusted Cox regression model was used to assess the relative risk of occurrence of the event between RA patients with depression and the matched population. Independent variables were the index depression status at index date (RA/depression and RA only) and patient characteristics.
The average number of occurrences per person-month attributable to depression was calculated among patients with at least one occurrence and reported at 6 and 12 months.
To assess the difference between the two groups (RA/depression and RA only), we also estimated a ratio between number of occurrences of health care utilization in the “RA/depression” group and in the “RA only”, by health care utilization category and among patients with at least one occurrence.
The incidence rate in turn was calculated based on the number of occurrences per patient-month. The average cost per person-month and cost attributable to depression were calculated and reported during different time periods: 6, 12 months, and the study period.
Adjusted generalized linear models with gamma distribution were fitted to compare the healthcare costs between RA patients with depression and the matched-controlled population in order to assess the impact of patients’ clinical and baseline characteristics. All analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC, USA). A P value of < 0.05 was considered statistically significant.
Results
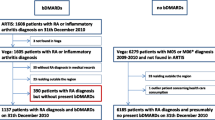
The patient flow chart for this analysis is described in Fig. 1. From a total of 91,669 RA patients, 8968 fulfilled the inclusion criteria. Among the included patients, 474 (5%) were affected with depression as a comorbidity.
The patient demographics before and after matching are summarized in Table 1. The subpopulation with depression comorbidity had a higher fraction of females and a higher Charlson comorbidity score in general.
Our findings also indicate that after matching, all observable differences between the two subpopulations (with and without depression) disappear (Table 1). The balance after matching is also evident from Fig. 2, which shows the distribution of the propensity score in the two groups before and after matching.
Table 2 presents the findings for the effect on health care utilization attributable to depression after 6 and 12 months. Within 6 and 12 months, most patients (> 98%) had at least one outpatient visit per month (including or excluding psychiatric outpatient visits). Depression as a comorbidity was associated with 62% higher total outpatient visits per month within 6 months (56% within 12 months). This difference was explained by more intervention of psychiatrists [(6 months): 55% of patients with depression vs. 2% of patients without depression; (12 months): 57 vs. 2%], more non-psychiatric specialist visits [(6 months): 2.5 vs. 1.9 visits per patient-month; (12 months): 2.2 vs. 1.7]. Results were confirmed by the adjusted Cox regression models: The relative risk of having a non-psychiatrist visit over the follow-up period was significantly higher in RA patients with depression than in RA patients without depression [(6 months): HR = 1.239 (1.116; 1.375), P < 0.0001; (12 months): HR = 1.257 (1.133; 1.395), P < 0.0001].
Within 6 months, the proportion of RA patients with at least one emergency room visit was 2.0% in patients with depression and 1.4% in patients without depression. Among them, the average number of emergency room visits were respectively 66 and 163% higher in RA patients affected with RA depression than without depression [(6 months): 1.96 vs. 1.18 (0.09); (12 months): 1.69 vs. 0.64]. The relative risk of an emergency room visit was higher in patients with depression than in patients without depression [HR = 2.059 (1.602; 2.646); P < 0.0001] (Table 3).
The proportion of patients with hospitalization was higher in patients with depression (14 vs. 7% within 6 months; 19 vs. 9% within 12 months). Within 6 months, patients were significantly more likely to have a hospitalization [HR = 1.474 (1.053; 2.062); P = 0.024]. Within 12 months, no significant difference in the relative risk of hospitalization were found [HR = 1.064 (0.802; 1.411); P = 0.668). Average numbers of visits per month among patients with at least one hospitalization were similar [(6 months): 0.22 vs. 0.23; (12 months) 1.69 vs. 0.64].
Within 6 months, the total resource expenditures were estimated at ¥ 116,068 per patient-month for RA patients with depression and ¥ 76,362 for RA patients without depression. The difference attributable to depression was ¥ 39,706 per patient-month and was divided equally between outpatient, inpatient, and pharmacy services (respectively, ¥ 15,420; ¥ 11,682; ¥ 13,017 per patient-month). Results were similar when considering resource utilization within 12 months: The difference in total expenditures per patient-month was similar within 12 months (¥ 32,022) with a comparable distribution between outpatient (¥ 12,735), inpatient (¥ 8004), and pharmacy (¥ 12,441) services (Table 4).
The results were validated by a means of an adjusted GLM regression model, which is reported in Table 5. The table shows that total outpatient costs, total inpatient costs per person-month, and pharmacy costs were significantly higher in patients with RA compared to patients without RA, whatever the period of assessment. The effect size is up to twice as big for the depression population.
Discussion
We analyzed an administrative hospital database and found that a co-morbidity of RA and depression causes significant economic burden. The sample in our database had a similar composition as the general RA population with a majority of the patients being female and middle-aged [16].
Our database identified only 5% of patients with RA affected by depression, which is considerably lower than reported estimates of other countries [4, 5] but exceeds the respective rate of the Japanese general population [17, 18]. There are two possible explanations for this finding. The possible differences in prevalence estimates between Japan and other developed countries may be in part due to a higher reluctance for reporting of depression in the Japanese population compared to other developed countries. In Japan, individuals affected with depression were reportedly less likely to seek medical treatment or consult a psychiatrist; 27% sought any treatment with only 14% having consulted a psychiatrist. This number is considerably lower than the reported estimates from several developed countries with approximately half that of the US [17]. The other possible explanation relates to the nature of our claims database; there is a bias towards patients employed at private Japanese companies and excludes patients unable to physically perform work-related tasks due to the severity of their disease. Accordingly, the depression prevalence identified from this database may have possibly underestimated the actual prevalence rate. Our estimated prevalence of 5% is in agreement with a recent survey that similarly found only 5% of the studied population had been formally diagnosed with depression. On the other hand, 35% of the affected patients demonstrated Patient Health Questionnaire (PHQ-9) scores indicative of the presence of depression, which translates to a significant underreporting of depression in RA patients. The PHQ-9 is a versatile self-reporting tool used for measurement of depression severity, diagnosis, as well as screening for the disease [19]. The study also found that among the RA population, younger patients, those affected by greater functional impairment, or patients on pain relief medications not biologic agents were more likely to experience depression [20]. Data from the Japanese IORRA registry also indicate that up to 41% of the RA patients were identified as depressed patients using a two-question screening tool [21], while a more recent study reported prevalence rates between 6.8 and 23.5% in depending on the tool that was used to determine depression [22].
In terms of health care utilization, depression resulted in a significant increase in number of outpatient visits and emergency room admissions. However, routine hospital admissions were not higher in the populations affected by depression. Only a limited number of studies have measured health care costs or resource utilization associated with depression comorbidity of RA patients. Findings from the US database studies revealed that the annual mean healthcare costs for patients with RA and depression comorbidity were USD 12,225 compared to USD 11,404 for patients with RA alone [23]. Our results indicate that the incremental burden of depression seems to be even higher in Japan compared to the US study. Clinical implication of this study is that Japanese rheumatologists need to become acutely aware of potential depression disorders during management of RA in their patients. A multidisciplinary treatment team approach is one potentially effective solution for achieving higher awareness of depression among RA patients. A second key resolution is to involve patients in decision-making. Recently, we showed that Japanese patients affected with RA have a preference for a collaborative role in medical decision-making, especially those affected with a greater disease burden [24].
Several limitations need to be discussed in this study. First, we could not identify clinical parameters and disease severity from the claims database. This is an inherent limitation of claims database analysis in general. Therefore, identifiable factors were limited to the characteristics of patients such as age, gender, and comorbidity as well as clinical characteristics related to the treatment. Furthermore, a common problem in any database analysis is the coding quality. Most of the time, either the hospital or treating physician designates medical codes that provide the highest reimbursement rates, which is why disease codes do not always reflect clinical reality [25]. Finally, depression has a significant impact on healthcare utilization not only in RA but also other disease conditions [26].
Conclusions
In summary, in light of the increased burden of depression among Japanese patients with RA, treating specialists need to be more vigilant and aware of the potential for comorbidity depression impacting their patients’ quality of life and ensure that the patients are actively involved in treatment decision-making. Future large clinical studies are required to examine if the additional burden of depression is greater in patients affected with RA compared to other diseases such as cancer.
References
Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64(1):52–60.
Löwe B, Willand L, Eich W, Zipfel S, Ho AD, Herzog W, et al. Psychiatric comorbidity and work disability in patients with inflammatory rheumatic diseases. Psychosom Med. 2004;66(3):395–402.
Isik A, Koca SS, Ozturk A, Mermi O. Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26(6):872–8.
Bruce TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep. 2008;10(3):258–64.
Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2013;52(12):2136–48.
Li X, Gignac MA, Anis AH. The indirect costs of arthritis resulting from unemployment, reduced performance, and occupational changes while at work. Med Care. 2006;44(4):304–10.
Margaretten M, Julian L, Katz P, Yelin E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumtol. 2011;6(6):617–23.
Sruamsiri R, Mahlich J, Tanaka E, Yamanaka H. Productivity loss of Japanese patients with rheumatoid arthritis—a cross-sectional survey. Mod Rheumatol. 2017. https://doi.org/10.1080/14397595.2017.1361893.
Nagyova I, Stewart RE, Macejova Z, van Dijk JP, van den Heuvel WJ. The impact of pain on psychological well-being in rheumatoid arthritis: the mediating effects of self-esteem and adjustment to disease. Patient Educ Couns. 2005;58(1):55–62.
Sleath B, Chewning B, de Vellis BM, Weinberger M, de Vellis RF, Tudor G, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum. 2008;59(2):186–91.
Mattey DL, Dawes PT, Hassell AB, Brownfield A, Packham JC. Effect of psychological distress on continuation of anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. J Rheumatol. 2010;37(10):2021–4.
Kuwabara H, Saito Y, Mahlich J. Adherence and re-hospitalizations in patients with schizophrenia: evidence from Japanese claims data. Neuropsychiatr Dis Treat. 2015;11:935–40.
Davis K, Meyers J, Zhao Z, McCollam P, Murakami M. High-risk atherosclerotic cardiovascular disease in a real-world employed Japanese population: prevalence, cardiovascular event rates, and costs. Atheroscler Thromb. 2015;22(12):1287–304.
Rosenbaum P, Rubin D. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–8.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Yamanaka H, Sugiyama N, Inoue E, Taniguchi A, Momohara S. Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Mod Rheumatol. 2014;24(1):33–40.
Kawakami N. Epidemiology of depressive disorders in Japan and the world. Jpn J Clin Med. 2007;65(9):1578–84.
Mahlich J, Tsukazawa S, Wiegand F. Estimating prevalence and health care utilization for treatment resistant depression in Japan: a retrospective claims database study. Drugs Real World Outcomes. 2017. https://doi.org/10.1007/s40801-017-0126-5.
Muramatsu K, Miyaoka H, Kamijima K, Muramatsu Y, Yoshida M, Otsubo T, et al. The patient health questionnaire, Japanese version: validity according to the Mini-International Neuropsychiatric Interview-Plus. Psychol Rep. 2007;101:952–60.
Sruamsiri R, Kaneko Y, Mahlich J. The underrated prevalence of depression in patients with rheumatoid arthritis: evidence from a nationwide survey in Japan. BMC Rheumatol. 2017;1:5.
Nakajima A, Kamitsuji S, Saito A, Tanaka E, Nishimura K, Horikawa N, et al. Disability and patient’s appraisal of general health contribute to depressed mood in rheumatoid arthritis in a large clinical study in Japan. Mod Rheumatol. 2006;16(3):151–7.
Sato E, Nishimura K, Nakajima A, Okamoto H, Shinozaki M, Inoue E, et al. Major depressive disorder in patients with rheumatoid arthritis. Mod Rheumatol. 2013;23(2):237–44.
Joyce AT, Smith P, Khandker R, Melin JM, Singh A. Hidden cost of rheumatoid arthritis (RA): estimating cost of comorbid cardiovascular disease and depression among patients with RA. J Rheumatol. 2009;36(4):743–52.
Mahlich J, Sruamsiri R. Preference for shared decision-making in Japanese patients with rheumatoid arthritis. Cogent Med. 2017;4:1353262.
Czwikla J, Domhoff D, Giersiepen K. ICD coding quality for outpatient cancer diagnoses in SHI claims data. Z Z Evid Fortbild Qual Gesundh wesen. 2016;118–119:48–55.
Manning W, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30:541–53.
Acknowledgements
Funding
We received funding from Janssen Pharmaceutical KK to conduct the survey. Article processing charges were funded by Janssen Pharmaceutical KK. The study design, data collection, data analysis, data interpretation, and writing of the report were solely completed by the authors. The findings and conclusions in this report do not necessarily reflect the views of Janssen Pharmaceutical KK. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and/or Editorial Assistance
Florent Guelfucci, Jörg Mahlich, Yuko Kaneko, and Rosarin Sruamsiri made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; Florent Guelfucci performed analysis; Jörg Mahlich, Rosarin Sruamsiri, and Yuko Kaneko have been involved in drafting the manuscript; Florent Guelfucci, Jörg Mahlich, Yuko Kaneko, and Rosarin Sruamsiri discussed for critical important intellectual content and revise accordingly; and Florent Guelfucci, Jörg Mahlich, Yuko Kaneko, and Rosarin Sruamsiri have given final approval of the version to be published. We would like to thank Dr. Negar Jamshidi for proofreading the manuscript.
Disclosures
Jörg Mahlich is an employee of Janssen Pharmaceutical KK. Rosarin Sruamsiri is an employee of Janssen Pharmaceutical KK. Yuko Kaneko has received lecture fees from AbbVie, Eisai Pharmaceutical, Chugai Pharmaceutical, Bristol Myers Squibb, Astellas Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Pfizer, Janssen, and UCB. Florent Guelfucci has received funding from Janssen KK to conduct the analysis.
Compliance with Ethics Guidelines
This was a retrospective study carried out using hospital claims data from the JMDC database; the authors were not directly involved in the collection of this data. Retrieval of the data from this database occurred in an unlinked fashion. As the data had been anonymized, the Ethical Guidelines for Epidemiological Research (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labor and Welfare of Japan), which require ethics approval and informed consent, are not applicable to this study. Based on the Ethical Guidelines on Biomedical Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labor and Welfare of Japan), pharmacoepidemiological studies conducted on medical databases constitute research carried out on pre-existing material and information, which did not require any interventions or interactions with patients. For such studies, including this study, obtaining written informed consent from patients is not compulsory.
Data Availability
The dataset supporting the conclusions is not publicly available. Interested readers may contact the corresponding author.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to https://doi.org/10.6084/m9.figshare.5849865.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Guelfucci, F., Kaneko, Y., Mahlich, J. et al. Cost of Depression in Japanese Patients with Rheumatoid Arthritis: Evidence from Administrative Data. Rheumatol Ther 5, 171–183 (2018). https://doi.org/10.1007/s40744-018-0096-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-018-0096-4