Abstract
Introduction
The efficacy of sarilumab and upadacitinib, in combination with disease-modifying antirheumatic drugs (DMARDs), was demonstrated in phase 3 clinical trials of patients with rheumatoid arthritis (RA) refractive to previous biologic DMARDs. In the absence of head-to-head clinical trials, the matching-adjusted indirect comparison (MAIC) and simulated treatment comparison (STC) estimate the relative efficacy of sarilumab and upadacitinib in patients with RA who had an inadequate response to previous biologic DMARDs.
Methods
Patient-level data for sarilumab were obtained from the TARGET trial (NCT01709578) and published aggregate data for upadacitinib were obtained from the SELECT-BEYOND trial (NCT02706847). For the MAIC, individual patient data from the TARGET trial were assigned weights such that weighted mean baseline characteristics of the treatment effect modifiers matched those from SELECT-BEYOND. For the STC, the TARGET patient-level data and mean baseline values from SELECT-BEYOND were used to simulate sarilumab treatment effects for a SELECT-BEYOND population. Endpoints evaluated included the American College of Rheumatology (ACR) response criteria ACR20/50/70, Disease Activity Score-28 for Rheumatoid Arthritis with C-reactive protein (DAS28-CRP) < 3.2, DAS28-CRP < 2.6, Simple Disease Activity Index (SDAI) < 3.3, and Clinical Disease Activity Index (CDAI) < 2.8 at 12 weeks.
Results
The analysis included 365 patients from TARGET and aggregated data of 333 patients from SELECT-BEYOND. Matching for potential treatment effect baseline modifiers (i.e., age, oral glucocorticoid use, tender joint count of 68 counts, swollen joint count of 66 counts, serum CRP level, and patient global assessment of disease activity) resulted in a reduction of the effective sample size of TARGET population to 166. Following MAIC and STC analysis, the odds of achieving all aforementioned clinical outcomes versus placebo at week 12 were similar for sarilumab and upadacitinib.
Conclusion
In the MAIC and STC analyses from TARGET and SELECT-BEYOND trials, the efficacy of sarilumab and upadacitinib were comparable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The interleukin (IL)-6 receptor inhibitor, sarilumab, and the Janus kinase (JAK)-1 inhibitor, upadacitinib, are approved for treatment of patients with moderately to severely active rheumatoid arthritis (RA), but head-to-head comparisons in clinical trials have not been performed to date. |
In the absence of head-to-head comparisons, and where there are only one or two trials for a treatment, approaches for the indirect comparisons of different treatments include the matching-adjusted indirect comparison (MAIC) and the simulated treatment comparison (STC). |
What did the study ask? What was the hypothesis of the study? |
We used the MAIC and STC analyses to estimate the relative efficacy of sarilumab and upadacitinib in patients with RA who had an inadequate response to previous biologic disease-modifying antirheumatic drugs (bDMARDs) using data from the TARGET (sarilumab) and SELECT-BEYOND (upadacitinib) trials. |
What was learned from the study? What were the study outcomes/conclusions? |
Our results, obtained using the two population-adjusted indirect comparisons (MAIC and STC), suggest a similar effect of sarilumab and upadacitinib when given in combination with conventional synthetic DMARDs. |
What has been learned from the study? |
To the best of our knowledge, this analysis was the first indirect comparison of sarilumab and upadacitinib, and one of the first studies in RA to utilize the STC methodology. |
Indirect comparisons have become increasingly common in assessing comparative efficacy; their use should be encouraged but critically evaluated. |
Introduction
Targeted disease-modifying antirheumatic drugs (DMARDs), biologic or synthetic, are commonly prescribed for patients with rheumatoid arthritis (RA) who have a suboptimal response to conventional synthetic DMARDs alone [1, 2]. As examples of the available options in RA, the interleukin (IL)-6 receptor inhibitor, sarilumab, administered as a subcutaneous injection, and the Janus kinase (JAK)-1 inhibitor upadacitinib, administered orally, are both approved for treatment of patients with moderately to severely active RA [3,4,5], but head-to-head comparisons in clinical trials have not been performed to date, particularly in patients who have failed biologic DMARDs (bDMARDs) previously.
In the absence of head-to-head trials, network meta-analyses can be used to evaluate effects of various treatments indirectly. However, in networks consisting of only one or two trials per treatment, indirect comparisons are highly vulnerable to systematic variation (bias) resulting from imbalances in effect modifier distributions [6, 7]. Alternative approaches for the indirect comparison of different treatments include the matching-adjusted indirect comparison (MAIC) and the simulated treatment comparison (STC). In the MAIC, a propensity score weight is used to adjust for differences between the individual patient-level data in the index trial and the comparator study by increasing the weight of individuals with more similar patient-related factors [8]. In the STC, patient-level data from one trial are used to model the outcome(s) of interest as a function of relevant covariates. A regression model is then used to predict the outcomes for the index population that would have been observed in populations from comparator studies for which only aggregate study-level data are available [9].
Here, we report the MAIC and STC analyses of sarilumab and upadacitinib (approved doses) outcomes using data from the two phase 3 trials conducted to date in patients with RA who were refractory to previous treatment with bDMARDs.
Methods
Trials and Patients
The TARGET (NCT01709578) [10] trial of sarilumab and the SELECT-BEYOND (NCT02706847) [11] trial of upadacitinib were randomized, placebo-controlled studies that enrolled adults with active RA who had received conventional synthetic DMARDs and had prior bDMARD exposure. TARGET randomized 546 patients with tender joint count (68 joints assessed; TJC68) of ≥ 8, swollen joint count (66 assessed; SJC66) of ≥ 6, C-reactive protein (CRP) concentration ≥ 8 mg/l, disease duration ≥ 6 months, and either inadequate response or intolerance to tumor necrosis factor inhibitors. The follow-up periods for its two co-primary endpoints were 12 weeks (change from baseline in the Health Assessment Questionnaire—Disability Index [HAQ-DI]) and 24 weeks (proportion of patients who achieved American College of Rheumatology criteria for 20% improvement [ACR20]) [10]. SELECT-BEYOND randomized 499 adults with similar inclusion criteria with minor differences: TJC68 and SJC66 ≥ 6, CRP ≥ 3 mg/l, and inadequate response or intolerance to ≥ 1 bDMARD. The follow-up for both co-primary endpoints – the proportion of patients achieving ACR20 and the proportion of patients who achieved a 28-joint disease activity score using CRP (DAS28-CRP) of ≤ 3.2—was 12 weeks [11].
The MAIC and STC analyses comprised patient-level data of participants using the recommended dose of sarilumab (200 mg q2w) from TARGET (index trial) and aggregate patient data using upadacitinib (15 mg/day) from SELECT-BEYOND (comparator trial), or placebo.
Outcomes
Endpoints evaluated included the proportions of patients achieving ACR20, ACR50, ACR70, DAS28-CRP < 3.2, DAS28-CRP < 2.6, Simple Disease Activity Index (SDAI) < 3.3, and Clinical Disease Activity Index (CDAI) < 2.8. Additional endpoints were changes from baseline to week 12 in DAS28-CRP, HAQ-DI, pain Visual Analog Scale (VAS), and Patient Global Assessment of Disease Activity (PtGA).
Statistical Analysis
In the MAIC [8], we identified covariates that were statistically significantly different at baseline. Based on clinical judgement, we excluded covariates that were likely associated with each other to avoid potential collinearity and unnecessary reduction in effective sample size. This first step identified the following variables: age, oral glucocorticoid use, TJC68, SJC66, CRP, and PtGA. A logistic propensity score model was used to estimate weights for the patient-level data from TARGET so that the weighted mean baseline characteristics matched those observed in SELECT-BEYOND. The effective sample size (ESS), defined as the number of non-weighted patients needed to calculate parameter estimates with the same precision as the weighted sample estimate, was calculated to test model validity. As with typical sample size, a large value is preferable to a small value, as the larger sample contains more information.
In the STC [9], the sarilumab treatment effect in SELECT-BEYOND was simulated based on the efficacy data from TARGET, using a regression model adjusted for the covariates of age, oral glucocorticoid use, TJC68, SJC66, CRP, and PtGA, centered at their mean values from SELECT-BEYOND. For consistency, the same covariates were selected as for the MAIC.
Treatment effects for the weight-adjusted (MAIC) and regression-simulated TARGET population (STC) at week 12 were compared with published aggregate data from SELECT-BEYOND via a Bucher indirect treatment comparison, thereby correcting for the placebo values [6]. An as-observed analysis was used to account for dropouts and early terminations.
For both the MAIC and STC, the placebo-corrected odds ratios (OR) of achieving discrete outcomes (sarilumab vs. upadacitinib) were calculated. Continuous parameters were also analyzed using placebo-corrected values. All statistical analyses were conducted using R, version 3.6.2 (packages base, stats, Hmisc, haven, utils, weights, questionr, and reldist) and SAS, version 9.4 (Cary, NC, USA). Both TARGET and SELECT-BEYOND studies were approved by the appropriate ethics committees/institutional review boards [10, 11].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
The analysis included 365 patients from TARGET and aggregated data of 333 patients from SELECT-BEYOND. Details of population screening and matching analyses are depicted in Fig. 1. Week 12 response rates and mean changes from baseline from the TARGET and SELECT-BEYOND trials in the outcome measures used in the MAIC and STC analyses described below are shown in Fig. S1 in the Supplementary Material.
Overall, patient populations in the two trials had comparable baseline characteristics.
For the MAIC, the parameters that had statistically significant differences between the TARGET sarilumab 200 mg q2w group and the SELECT-BEYOND upadacitinib 15 mg/day group were: age, previous treatment with at least one TNFα inhibitor, oral glucocorticoid use, SJC66, PtGA, pain VAS, DAS28-CRP and CRP (Table 1). Of these, we chose age (TARGET, 52.4 years; SELECT-BEYOND, 57.0 years), oral glucocorticoid use (62 vs. 47%), SJC66 (20.1 vs. 16.7), PtGA (70 vs. 67 mm), and CRP (28.4 vs. 16.2 mg/l) as covariates for the MAIC (Table 2). In addition, we added TJC68 (29.5 vs. 28.2) to the list of MAIC covariates, since TJC28 (a reduced version of TJC68) is a component of DAS28. In both trials, response was similar regardless of number of TNFα inhibitors, hence we excluded this potential covariate [12, 13]. After weighting for the MAIC analysis, the mean ± SD values of age, TJC68, SJC66, and CRP, and percentage with oral glucocorticoid use, in patients from TARGET became identical to those from SELECT-BEYOND. In this process, the ESS of TARGET was reduced to 46% (166/365) of the initial population (sarilumab, 92; placebo, 74). In STC, baseline characteristics were adjusted via a regression equation instead of a propensity score matching approach.
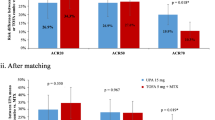
After both the MAIC and STC, the odds of achieving each and every composite clinical outcome were not significantly different between sarilumab and upadacitinib (Fig. 2a, b). The odds of achieving CDAI < 2.8 did not differ significantly between the two DMARDs (MAIC OR = 10.8, 95% CI 0.0 – 6597.6; STC OR = 6.3, CI 0.2–170.4), which was also the case for DAS28-CRP < 2.6 (MAIC OR = 0.7, CI 0.1–3.3; STC OR = 4.2, CI 0.9–19.3), DAS28-CRP < 3.2 (MAIC OR = 0.95, CI 0.3–3.7; STC OR = 1.8, CI 0.5–6.0), ACR70 (MAIC OR = 1.2, CI 0.2–8.8; STC OR = 4.6, CI 0.7–31.6), ACR50 (MAIC OR = 0.5, CI 0.1–2.0; STC OR = 1.0, CI 0.3–3.1), and ACR20 (MAIC OR = 0.6, CI 0.2–1.8; STC OR = 0.7, CI 0.3–1.7).
Odds ratio (sarilumab vs. upadacitinib, placebo-corrected) of achieving various composite clinical outcomes. aThe OR calculation of attaining SDAI < 3.3 in the MAIC analysis was not performed because the estimate of the number of placebo-treated patients with such a score was zero. bIn the MAIC, the effective sample size of TARGET was reduced to 46% (166/365) of the initial population (sarilumab, n = 92; placebo, n = 74). cIn the STC, by design, baseline characteristics were adjusted via a regression equation instead of a propensity score matching approach. ACR 20/50/70 American College of Rheumatology response criteria, CDAI Clinical Disease Activity Index, CI confidence interval, DAS28-CRP Disease Activity Score-28 for Rheumatoid Arthritis with C-reactive protein, MAIC matching-adjusted indirect comparison, SDAI Simple Disease Activity Index, STC simulated treatment comparison
These findings were mirrored in all continuous outcomes evaluated, as evidenced by no appreciable differences detected between the treatment effects of sarilumab and upadacitinib (Fig. 3). There was no significant difference (least squares mean difference [LSMD]) in score change between treatments for DAS28-CRP (MAIC 0.1, 95% CI − 0.6 to 0.8; STC − 0.2, CI − 0.7 to 0.4 [Fig. 3a]) and HAQ-DI (MAIC 0.1, CI − 0.2 to 0.3; STC 0.0, CI − 0.2 to 0.3 [Fig. 3b]). Further, there was no significant difference (LMSD) in pain VAS change between the treatments (MAIC − 0.3, CI − 14.7 to 14.1; STC − 0.2, CI − 11.2 to 10.8 [Fig. 3c]).
Difference in score changes in continuous outcomes from baseline to week 12, sarilumab minus upadacitinib (placebo-corrected)a. aIn the MAIC, the effective sample size of TARGET was reduced to 46% (166/365) of the initial population (sarilumab, n = 92; placebo, n = 74). In the STC, by design, baseline characteristics were adjusted via a regression equation instead of a propensity score matching approach. CI confidence interval, DAS28-CRP Disease Activity Score-28 for Rheumatoid Arthritis with C-reactive protein, HAQ-DI Health Assessment Questionnaire—disability index, LSMD least squares mean difference, MAIC matching-adjusted indirect comparison, STC simulated treatment comparison, VAS visual analog scale
Discussion
These data suggest that, at 12 weeks, the efficacy of sarilumab, an IL-6 receptor blocker, is comparable to that of upadacitinib, a JAK antagonist, in patients with RA who had an inadequate response to one or more previous bDMARD treatments and who were currently receiving conventional synthetic DMARDs.
In the absence of a head-to-head randomized controlled trial, this indirect comparison between sarilumab and upadacitinib in combination with conventional synthetic DMARDs may be meaningful, as similar results were obtained using two different methodologies. Since the relative reduction of sample size in studies using MAIC analysis was shown to decrease by 74% [14], and by as much as 80% [7, 15], our model, with the sample size reduction of only 54%, is less problematic. Furthermore, reduction in effective sample size in MAIC is considered to be less of a limitation than meta-regression, in which the number of trials, rather than the number of patients, is required to exceed the number of baseline characteristics used for adjustment [16]. However, it should be noted that only a properly powered, randomized, head-to-head study of the two molecules could truly establish non-inferiority.
To the best of our knowledge, this analysis was the first indirect comparison of sarilumab and upadacitinib, and one of the first studies in RA to utilize the STC methodology. With the proliferation of available targeted therapies for RA, and with a dearth of head-to-head randomized trials, comparing treatment effects indirectly using statistical methods is becoming increasingly performed. Recently, two studies compared RA therapies using a MAIC: one assessed the effects of upadacitinib against those of another JAK inhibitor, tofacitinib [17], and the other compared the JAK inhibitor baricitinib with tofacitinib, the TNFα inhibitor adalimumab, and the IL-6 receptor inhibitor tocilizumab [12]. Both studies used approaches similar to ours, with some differences in the adjustment factors. The comparison of upadacitinib and tofacitinib was conducted by weighing data from the upadacitinib trial SELECT-MONOTHERAPY based on age, sex, race, and the baseline values of SJC66/28, TJC68/28, CRP, and patients’ global assessment [17]; the study concluded that upadacitinib was associated with improved outcomes versus tofacitinib. The four-treatment comparison was conducted by adjusting data from the baricitinib trial RA-BEGIN based on age, sex, and the baseline scores of DAS28-ESR, pain VAS, and HAQ-DI [12]; in this study, greater pain reduction and improved physical function were found for baricitinib with adalimumab and tocilizumab, but not for baricitinib versus tofacitinib. A careful selection of adjustment factors is important; while these are selected based on subject matter expertise, it may be possible to incorrectly include some factors or omit others. Using factors that are not treatment effect modifiers, or missing a treatment effect modifier, influences how precise the analysis is and may introduce bias [18].
Although indirect comparison techniques based on population adjustment offer advantages over network meta-analyses (primarily in the fact that they do not assume equivalence of baseline characteristics), they are associated with certain limitations. In the case of the MAIC, the key limitation is the reduction of the ESS [8], and in the case of both the MAIC and STC (and the population adjustment analyses in general), one cannot adjust for potential modifiers that have not been captured in the study or are unavailable in published manuscripts, such as the conventional synthetic DMARD and dose a patient was receiving, or the number of prior bDMARD failures, or certain comorbidities (e.g., fibromyalgia) that may influence treatment response. A further limitation of this analysis is that lack of patient-level data for both studies meant that we could not perform univariable and multivariable logistic regression analyses to identify factors that pertained to (prior) treatment resistance in each treatment group. To confirm our findings, novel modeling methods such as multilevel network meta-regression for population-adjusted treatment comparisons [19] could be used. An effectiveness analysis, using a similar approach to compare studies of real-world data, would also be a possibility. However, it should be noted that use of each of these methods is hypothesis-generating rather than conclusive evidence.
Conclusions
Our results, obtained using two population-adjusted indirect comparisons, suggest a similar effect of sarilumab and upadacitinib when given in combination with stable conventional synthetic DMARDs. Indirect comparisons have become increasingly common in assessing comparative efficacy; their use should be encouraged but critically evaluated.
References
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1–25.
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Winthrop KL, Weinblatt ME, Bathon J, Burmester GR, Mease PJ, Crofford L, et al. Unmet need in rheumatology: reports from the Targeted Therapies meeting 2019. Ann Rheum Dis. 2020;79(1):88–93.
KEVZARA (sarilumab) Prescribing Information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf. Accessed 20 Jan 2022.
RINVOQ (upadacitinib) Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211675s000lbl.pdf. Accessed 20 Jan 2022.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91.
Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–11.
Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–45.
Caro JJ, Ishak KJ. No head-to-head trial? Simulate the missing arms. Pharmacoeconomics. 2010;28(10):957–67.
Fleischmann R, van Adelsberg J, Lin Y, Castelar-Pinheiro GD, Brzezicki J, Hrycaj P, et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2017;69(2):277–90.
Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–24.
Fautrel B, Zhu B, Taylor PC, van de Laar M, Emery P, De Leonardis F, et al. Comparative effectiveness of improvement in pain and physical function for baricitinib versus adalimumab, tocilizumab and tofacitinib monotherapies in rheumatoid arthritis patients who are naïve to treatment with biologic or conventional synthetic disease-modifying antirheumatic drugs: a matching-adjusted indirect comparison. RMD Open. 2020;6(1): e001131.
Weinblatt M, Thomson G, Chen K, Meerwin S, Schlacher C, Cush J. Clinical responses in patients with inadequate response to bDMARDs upon treatment with upadacitinib [abstract]. Arthritis Rheumatol. 2019;71(suppl 10). (abstract no 515).
Phillippo D, Dias S, Elsada A, Ades AE, Welton NJ. Population adjustment methods for indirect comparisons: a review of national institute for health and care excellence technology appraisals. Int J Technol Assess Health Care. 2019;35(3):221–8.
Phillipo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–11. https://doi.org/10.1177/0272989X17725740.
Signorovitch J, Sikirica V, Erder M, Xie J, Lu M, Hodgkins P, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–7.
Edwards CJ, Sawant R, Garg V, Du EX, Friedman A, Betts KA. A Matching-adjusted indirect comparison of upadacitinib versus tofacitinib in adults with moderate-to-severe rheumatoid arthritis. Rheumatol Ther. 2021;8(1):167–81.
Weber D, Jensen K, Kieser M. Comparison of methods for estimating therapy effects by indirect comparisons: a simulation study. Med Decis Making. 2020;40(5):644–54.
Phillippo DM, Dias S, Ades AE, Belger M, Brnabic A, Schacht A, et al. Multilevel network meta-regression for population-adjusted treatment comparisons. J R Stat Soc Ser A Stat Soc. 2020;183(3):1189–210.
Acknowledgements
We thank the participants of the TARGET and SELECT-BEYOND trials.
Funding
This study was funded by Sanofi. Publication of this article and the journal’s Rapid Service Fee were also funded by Sanofi.
Medical Writing/Editorial Assistance
Medical writing assistance, funded by Sanofi, was provided by Vojislav Pejović, PhD, Richard J. Hogan, PhD, and Rania Kairouz-Wahbe, PhD, of Elevate Medical Affairs, a division of Envision Pharma Group.
Author Contributions
Thomas Huizinga, Ernest Choy, Amy Praestgaard, Hubert van Hoogstraten, Patrick R. LaFontaine, Patricia Guyot, Daniel Aletaha, Ulf Müller-Ladner, Jeffrey R. Curtis and Roy Fleischmann were all involved in the design of the study, collecting and interpreting the data, the drafting of the manuscript, and critical revisions. Statistical analysis was done by Amy Praestgaard. The final version of the manuscript was approved by all authors, who had full access to all of the data in the study and had final responsibility for the decision to submit the manuscript for publication.
Prior Presentation
These data were presented at the American College of Rheumatology Convergence, Virtual, November 5–9, 2020 (Abstract 0827).
Disclosures
Thomas Huizinga has received grants and/or consultant fees from Ablynx, Bristol Myers Squibb, Roche, and Sanofi. Ernest Choy has received grant/research support (Amgen, Bio-Cancer, Chugai Pharma, Pfizer, Roche, UCB); consulting fees (AbbVie, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai Pharma, Eli Lilly, GlaxoSmithKline, Hospita, Ionis, Jazz Pharmaceuticals, Janssen, Merck Serono, Novartis, Novimmune, ObsEva, Pfizer, R-Pharm, Regeneron Pharmaceuticals, Inc, Roche, SynAct Pharma, Sanofi-Genzyme, UCB); and speaking fees (Amgen, Bristol Myers Squibb, Chugai Pharma, Eli Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc, Roche, Sanofi-Genzyme, UCB). Amy Praestgaard, Hubert van Hoogstraten, Patrick R. LaFontaine, and Patricia Guyot are employees of Sanofi and may own company stock. Daniel Aletaha has received grant/research support, consulting fees, and/or speaker fees/honoraria from AbbVie, Amgen, Celgene, Lilly, Medac, Merck, Novartis, Pfizer, Roche, Sandoz, Sanofi Genzyme, and UCB. Ulf Müller-Ladner has acted as speaker/advisor for AbbVie and Sanofi. Yoshiya Tanaka has received speaking fees and/or honoraria from Daiichi-Sankyo, Eli Lilly, Novartis, YL Biologics, Bristol Myers Squibb, Eisai, Chugai, Abbvie, Astellas, Pfizer, Sanofi, Asahi-kasei, GSK, Mitsubishi-Tanabe, Gilead, Janssen and has received research grants from Mitsubishi-Tanabe, Chugai, Abbvie, Takeda, UCB, Daiichi-Sankyo, Eisai. Jeffrey R. Curtis has received consulting and research grants from AbbVie, Amgen, BMS, Gilead, GSK, Janssen, Lilly, Myriad, Pfizer, Roche, Samsung, Sandoz, Sanofi, UCB. Roy Fleischmann has received research grants and/or consulting fees from AbbVie, ACEA, Amgen, AstraZeneca, Bristol Myers Squibb, Centrexion, Eli Lilly, EMD Serono, Genentech, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Samsung, Sandoz, Sanofi Genzyme, Selecta, and UCB.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Qualified researchers may request access to patient-level data and related documents (including, e.g., the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and data set specifications). Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies and process for requesting access can be found at: https://vivli.org.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huizinga, T., Choy, E., Praestgaard, A. et al. Clinical Efficacy of Sarilumab Versus Upadacitinib Over 12 weeks: An Indirect Treatment Comparison. Rheumatol Ther 10, 539–550 (2023). https://doi.org/10.1007/s40744-022-00521-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00521-1