Abstract
Purpose of review
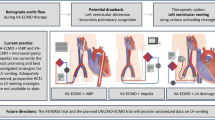
Cardiac extracorporeal membrane oxygenation (ECMO) for single ventricle heart disease is a complex intervention applied to a heterogeneous population of children. In this review, we describe the variable single ventricle populations who may be supported with ECMO and highlight recent literature regarding outcomes, trends, and evolving strategies.
Recent findings
In the last decade, there has been a significant increase in the international volume of single ventricle cardiac ECMO, with an overall trend towards improved survival. Single ventricle ECMO has now been accepted as a viable modality in most international centers, with an overall survival approximating 45% in the modern era. Single ventricle cardiac disease, with or without the requirement for ECMO, has an important impact upon long-term health-related quality of life outcomes.
Summary
While survival is slightly lower for single ventricle ECMO than in children with biventricular physiology, the need for ECMO identifies a subset of single ventricle patients who are at increased risk for interstage mortality, adverse neurodevelopmental outcomes, and the early onset of advanced heart failure symptoms. Care for survivors after ECMO requires increased surveillance and risk assessment prior to future intervention.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tweddell J, Sleeper L, Ohye R, et al. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–9.
Alsoufi B, McCracken C, Ehrlich A, Mahle W, Kokon B, Border W, et al. Single ventricle palliation in low weight patients is associated with worse early and midterm outcomes. Ann Thorac Surg. 2015;99:668–76.
Alsoufi B, McCracken C, Schlosser B, Sachdeva R, Well A, Kokon B, et al. Outcomes of multistage palliation of infants with functional single ventricle and heterotaxy syndrome. J Thorac Cardiovasc Surg. 2016;151:1369–77.
Tabbutt S, Ghanayem N, Ravishankar C, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–95.
Norwood WI, Lang P, Casteneda AR, Campbell DN. Experience with operations for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 1981;82:511–9.
Sano S, Ishino K, Kawada M, Arai S, Kasahara S, Asai T, et al. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:504–9.
Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–8.
D’Udekem Y, Iyengar A, Cochrane A, Grigg L, Ramsay J, Wheaton G, et al. The Fontan Procedure. Contemporary techniques have improved long term outcomes. Circulation. 2007;116:I–157– I-164.
• Ohye R, Schranz D, D’Udekem Y. Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation. 2016;134:1265–79 An in-depth review of evolving surgical therapies for hypoplastic left heart syndrome, describing how recent innovations have contributed to improving survival. Late outcomes for the Fontan population are considered in terms of quality of life and burdens of disease.
D’Udekem Y, Iyengar A, Galati J, et al. Redefining expectations of long-term survival after the Fontan procedure. Circulation. 2014;130:S32–8.
Mascio C, Austin E, Jacobs J, Jacobs M, Wallace A, He X, et al. Perioperative mechanical circulatory support in children: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. 2014;147(2):658–65.
Extracorporeal Life Support Organization. ECLS Registry Report: International Summary, Ann Arbor, MI; 2019. Available at: http://www.elso.org. Accessed 5 May 2019.
Sherwin E, Gauvreau K, Scheurer M, Rycus P, Salvin J, Almodovar M, et al. Extracorporeal membrane oxygenation after stage 1 palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2012;144:1337–43.
•• Ford M, Gauvreau K, McMullan M, Almodovar M, Cooper D, Rycus P, et al. Factors associated with mortality in neonates requiring extracorporeal membrane oxygenation for cardiac indications: analysis of the Extracorporeal Life Support Organization Registry Data. Pediatr Crit Care Med. 2016;17:860–70 Retrospective cohort study using ELSO registry data from 4471 neonates who required cardiac ECMO (2001–2011). Survival to hospital discharge was 41% with factors associated with mortality including lower birth weight, single ventricle heart disease, duration of ventilation, and severity of acidosis prior to ECMO. Survival was infrequent when ECMO was required for longer than 7 days.
D’Udekem Y, Shime N, Lou S, MacLaren G. Recurrent or prolonged mechanical circulatory support. Pediatr Crit Care Med. 2013;14:S69–72.
DeBrunner M, Porayette P, Breinholt J, Turrentine M, Cordes T. Midterm survival of infants requiring postoperative extracorporeal membrane oxygenation after Norwood palliation. Pediatr Cardiol. 2013;34:570–5.
Johansen A, Chiletti R, Best D. The impact of extracorporeal life support on hypoplastic left heart syndrome patients long term survival: a 10-year experience. Australian Critical Care. 2018;31:121.
Iguchi A, Ridout D, Galan S, Bodlani C, Squire K, O’Callaghan M, et al. Long-term survival outcomes and causes of late death in neonates, infants, and children treated with extracorporeal life support. Pediatr Crit Care Med. 2013;14:580–6.
Malik S, Bird TM, Jaquiss RD, et al. Comparison of in-hospital and longer-term outcomes of hybrid and Norwood stage 1 palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2015;150:474–80.
Roeleveld PP, Wilde RD, Hazekamp M, et al. Extracorporeal membrane oxygenation in single ventricle lesions palliated via the hybrid approach. World J Pediatr Congenit Heart Surg. 2014;5:393–7.
Newburger JW, Sleeper LA, Frommelt PC, et al. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation. 2014;129:2013–20.
Jolley M, Yaralagadda V, Rajagopal S, Almodovar M, Rycus P, Thiagarajan R. Extracorporeal membrane oxygenation–supported cardiopulmonary resuscitation following stage 1 palliation for hypoplastic left heart syndrome. Pediatr Crit Care Med. 2014;15:538–45.
Jaggers J, Forbess J, Shah A, Meliones J, Kirshbom P, Miller C, et al. Extracorporeal membrane oxygenation for infant postcardiotomy support: significance of shunt management. Ann Thorac Surg. 2000;69:1476–83.
Glenn W. Circulatory bypass of the right side of the heart: shunt between superior vena cava and distal right pulmonary artery. N Engl J Med. 1958;259:117–20.
Azzolina G, Eufrate S, Pensa P. Tricuspid atresia: experience in surgical management with a modified cavopulmonary anastomosis. Thorax. 1972;27:111–5.
Jolley M, Thiagarajan R, Barrett C, Salvin J, Cooper D, Rycus P, et al. Extracorporeal membrane oxygenation in patients undergoing superior cavopulmonary anastomosis. J Thorac Cardiovasc Surg. 2014;148:1512–8.
Fernandez R, Joy B, Allen R, Stewart J, Miller-Tate H, Miao Y, et al. Interstage survival for patients with hypoplastic left heart syndrome after ECMO. Pediatr Cardiol. 2017;38:50–5.
• Mahle W, Hu C, Trachtenberg F, et al. Heart failure after the Norwood procedure: an analysis of the Single Ventricle Reconstruction trial. J Heart Lung Transplant. 2018;37:879–85 Subjects enrolled in the Single Ventricle Reconstruction (SVR) trial were assessed at 6-year follow-up for adverse outcomes related to heart failure. 14% of children (n = 66) who survived to hospital discharge following Norwood operation suffered advanced heart failure, with death being the more likely outcome than heart transplantation.
Rood K, Teele S, Barrett C, Salvin J, Rycus P, Fynn-Thompson F, et al. Extracorporeal membrane oxygenation support after the Fontan operation. J Thorac Cardiovasc Surg. 2011;142:504–10.
Valeske K, Yerebakan C, Mueller M, Akintuerk H. Urgent implantation of the Berlin Heart Excor biventricular assist device as a total artificial heart in a patient with single ventricle circulation. J Thorac Cardiovasc Surg. 2014;147:1712–4.
Rossano JW, Kim JJ, Decker JA, et al. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J Card Fail. 2012;18:459–70.
Foulks M, Meyer R, Gold J, Herrington C, Kallin K, Menteer J. Postoperative heart failure after stage 1 palliative surgery for single ventricle cardiac disease. Pediatr Cardiol. 2019;40:943–9.
•• Burstein D, Shamszad P, Dai D, Almond C, Price J, Lin K, et al. Significant mortality, morbidity and resource utilization associated with advanced heart failure in congenital heart disease in children and young adults. Am Heart J. 2019;209:9–19 A retrospective analysis of advanced heart failure in children with congenital heart disease presenting to tertiary care pediatric hospitals in the United States (2004–2015). Single ventricle heart disease was strongly associated with mortality. Increasing utilization of ECMO, VAD, and heart transplantation are described over time.
De Rita F, Hasan A, Haynes S, Crossland D, Kirk R, Ferguson L, et al. Mechanical cardiac support in children with congenital heart disease with intention to bridge to heart transplantation. Eur J Cardiothorac Surg. 2014;46:656–62.
Blume E, VanderPluym C, Lorts A, et al. Second annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) report: pre-implant characteristics and outcomes. J Heart Lung Transplant. 2018;37:38–45.
Hetzer R, Kaufmann F, Delmo Walter EM. Paediatric mechanical circulatory support with Berlin Heart EXCOR: development and outcome of a 23-year experience. Eur J Cardio-Thor Surg. 2016;50:203–10.
Marino B, Lipkin P, Newberger J, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management. Circulation. 2012;126:1143–72.
Costello J, O’Brien M, Wypij D, Shubert J, Salvin J, Newberger J, et al. Quality of life of pediatric cardiac patients who previously required extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2012;13:428–34.
• Sadhwani A, Cheng H, Stopp C, Rollins C, Jolley M, Dunbar-Masterson C, et al. Early neurodevelopmental outcomes in children supported with ECMO for cardiac indications. Pediatr Cardiol. 2019;40:1072–83 Neurodevelopmental outcomes for cardiac ECMO survivors are described at 12–42 months of age. Cardiac ECMO survivors have significant neurodevelopmental delays which require follow-up and early intervention.
Ryerson L, Guerra G, Joffe A, et al. Survival and neurocognitive outcomes after cardiac extracorporeal life support in children less than 5 years of age. Circ Heart Fail. 2015;8:312–21.
Van Zellem L, Buysse C, Madderom M, Legerstee J, Aarsen F, Tibboel D, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015;41:1057–66.
Ravishankar C, Zak V, Williams I, et al. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the Pediatric Heart Network Infant Single Ventricle trial. J Pediatr. 2013;162:250–6.
Goldberg C, Lu M, Sleeper L, et al. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr. 2014;165:490–6.
Friesland-Little J, Uzark K, Yu S, Lowery R, Aiyagari R, Hirsch-Romano J. Functional status and quality of life in survivors of extracorporeal membrane oxygenation after the Norwood operation. Ann Thorac Surg. 2017;103:1950–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Bennett Sheridan, Warwick Butt, and Graeme MacLaren declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology/CT Surgery
Rights and permissions
About this article
Cite this article
Sheridan, B., Butt, W. & MacLaren, G. ECMO in Single Ventricle Heart Disease. Curr Treat Options Peds 5, 356–367 (2019). https://doi.org/10.1007/s40746-019-00173-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-019-00173-4