Abstract
Extracting, transportation and the using from fossil fuels can damage to the hydrosphere, the biosphere and the Earth’s atmosphere. But humans always need to this valuable substance. The production of oil derivatives by means of forest waste and coal through the Fischer–Tropsch process is an appropriate solution for the cleanliness of all parts of the environment. For the production of favorite products by the synthesis of Fischer–Tropsch, the performance of the catalyst under different operating conditions should be predictable. For this reason, in this paper, eight mathematical models were determined for the selectivity of five products of methane, light hydrocarbons, gasoline, diesel and wax based on three factors of reduction temperature, time on stream, and H2/CO ratio inlet gas on iron-based catalyst. The results showed that the reduction temperature factor had the most effective on the selectivity of hydrocarbon products, exception diesel, so that the increase of the reduction temperature led to increase of the selectivity of methane, light hydrocarbons, gasoline and reduce of the degree of selectivity of the wax and vice versa. For the diesel selectivity, factor of the H2/CO ratio inlet gas was the most effective than other factors.
Similar content being viewed by others
1 Introduction
Reducing the use of fossil fuels is one of the ways to prevent atmospheric and lithospheric pollutions. The fossil fuels have caused irreparable damage to all parts of the environment by generation of the greenhouse gas. But it is undeniable the human need for this valuable substance. Therefore, the production of fuel should be somehow that: (1) minimizes damage to the environment; (2) is available; (3) provides the necessary energy; (4) conforms to existing infrastructure; it is always a concern for all of the many politicians and scientists in the world. One of the activities undertaken in this field is the production of oil derivatives using Fischer–Tropsch (FT) process (FTP) (Aitken et al. 2016). Fischer–Tropsch synthesis is a catalytic process that its raw materials produce (H2, CO, CO2) from biosphere (forest waste and coal) (González-Delgado et al. 2015; Lueking and Cole 2017; Domínguez-García et al. 2017; Landau et al. 2014; Weckhuysen 2013). For this reason, the use of the FTP in areas suffering from supply lack of fossil fuels (Europe and South America) is a very good way to produce fuel. FTP produces the oil derivatives with high quality, (without aromatic and devoid of sulfur) (Xinling and Zhen 2009; Zhang et al. 2014) during the reactions (1–3) (Abelló and Montané 2011):
The products of FTP are dependent on catalyst and operational conditions applied to the process (Silva et al. 2008). Traditionally, for optimal production in this process, have been used cobalt and iron catalysts (Noureldin et al. 2014; Liu et al. 2014). When the synthesis gas (H2, CO, CO2) is extracted from coal and waste of forest, the H2/CO ratio is lower. In this case, iron-based catalyst is used to produce more products (Zhang et al. 2010). The iron catalyst provides the additional hydrogen for the FT synthesis by the water–gas shift reaction (WGS), thereby is avoided the accumulation of carbon at the catalyst level, as a result the iron-based catalyst is stably. To improve the performance of the catalyst, promoters are used. Promoters cause the increase of stability, activity, and selectivity of the catalyst with developing active sites on its the surface (Ayodele et al. 2017). The most popular promoters for iron catalyst, can mention Cu and K. Cu facilitates the reduction of iron catalyst, increases the absorption of H2, but heavily suppressed CO adsorption and carburization. On the other hand, K absorbs CO and promotes carburization of the iron catalyst (Li et al. 2015). The presence of both Cu and K promoters improve the performance of the WGS reaction and FTS (Ma et al. 2014; Wan et al. 2008). The supports are as the catalysts structural booster. When the FTS catalyst does not have a structural booster, it suffers from poor flexibility, low activity, and sintering. Among the structural booster, Si and Al can be pointed out, which they can stop sintering and increase the active phase stabilize and physical resistance. Si has a high carbonization capability, thus better absorbs CO. In the study of the two supports effect (SiO2 and Al2O3) on iron based catalyst with presence of potassium, Wu Bao Shan pointed out that SiO2 increased the activity of FTS and WGS and whereas Al2O3 decreased them (Rafati et al. 2015; Suo et al. 2012; Wan et al. 2007). The collection of iron, potassium and copper on silica, are achieved a catalyst which has high activity and a very good structure for the FT synthesis. Fe/Cu/K/SiO2 is an important industrial catalyst which various studies have been conducted at different weight percentages (Bukur et al. 2005; Bukur and Sivaraj 2002; Ma et al. 2016; Vengsarkar et al. 2015; Todic et al. 2016). Bukur researched the effect of reaction conditions on the selectivity of olefin by this catalyst. He reported increase of the pressure and temperature of reaction have a positive effect on the products selectivity (Bukur et al. 1997). In addition, Qing examined the importance of reduction conditions, including temperature, pressure, and the type of gas used in the reduction, on selectivity of products by Fe/Cu/K/SiO2 catalyst (Hao et al. 2007). Many studies indicate how reaction conditions influence on the degree of selectivity of products (Hao et al. 2007; Bukur et al. 1999; Duvenhage and Coville 2002; Ngantsoue-Hoc et al. 2002). However, none of the studies has provided a model so that expresses the significance of these factors. Also, the effect of different parameters on the FT reaction and the interaction between those parameters can be explored. For this purpose, the response surface methodology (RSM) is used. RSM is a set of mathematical relations that can describe the performance of a catalyst under different operating conditions and by means of that the optimum conditions are achieved. The aim of the study is to achieve a selectivity model for the products of the FT process under the iron-based catalyst (Fe–Cu–K/SiO2) in the operating conditions applied in Hao et al. (2007).
2 Experimental
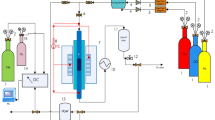
The used catalyst was prepared by mixing the three metals—Fe, Cu, K— and on the SiO2 support through drying-spraying technique.
2.1 Preparation of the catalyst
Fe (NO3)3 and Cu (NO3)2 were mixed in a dish with silica gel solution, then the solution sedimented. Following sedimentation, sodium carbonate solution was added to the sediment for stabilization of pH at 9 ± 0.1. So, the sediment was filtered and washed. To make K/Fe ratio 6/100, K2NO3 solution and distilled water were added to the sediment. The resulting cake was taken out, dried at 250 °C at 2.5 MPa, spray-dried, and crushed into powder. The obtained powder had a regular spherical form and suitable physical resistance for FTS slurry. Calcination temperature was 320 °C for 5 h. Eventually, a catalyst with the weight percentage of 100Fe/5Cu/6K/16SiO2 was made. The reduction conditions for this catalyst were studied at different temperatures. The area of BET, volume, and mean size of the pores of the catalyst at different temperature reduction (TR) are given in Table 1.
2.2 Experiment with the catalyst
To perform Fischer–Tropsch process, a stirred tank slurry reactor was used. Almost 20 g of the catalyst was placed inside the reactor to examine the effect of TR on the performance of the catalyst. Afterwards, the catalyst was reduced at the different temperatures (240–280 °C), pressure of 0.4 MPa, and synthesis gas (H2/CO = 0.67) for 13 h. The experiments were conducted under the following conditions: reaction temperature of 250 °C, the pressure of 1.5 MPa, and the specific velocity of 2 NL/g-cat h. To identify the products, the gas chromatography (GC) device was used. Using the data obtained from GC and the mathematical relations (that will have provided), the model of selectivity of the products are determined. The products are mainly methane, light hydrocarbons, gasoline, diesel, and wax. As previously stated, this study focuses on determination a comprehensive model for the selectivity of products.
3 Response surface methodology
To determine a favourable model, the effect of each parameter and their interactions are required. Among all the multivariate models for optimization analytical, the response surface methodology is the best—it is a powerful mathematical and statistical technique for developing, improving, and optimizing of the conditions in multivariate systems. Furthermore, it has the ability to formulate new products and the effect of independent variables and their interaction in the process. In this technique, the general relationship between the response and the input data is depicted in Eq. (4).
where \(\uppsi\) is the dependent variable or response, g is an unknown complex function of the independent variables \(x_{1} ,x_{2} ,x_{3} , \ldots ,x_{n}\), n is the number of input data, and \(\upvarepsilon\) is the fitting error, often assuming it to have a normal distribution with mean zero.
The steps of RSM as an optimization method are as follows:
-
(1)
The first step defines the independent variables of major effects on the reaction system via screening experiments, because it is not possible to identify the effects of all parameters.
-
(2)
In the second step, the experiments are designed according to tested characteristics such as selection of experimental points and the number of runs and blocks.
-
(3)
In the third stage, the independent parameters are made dimensionless. Each effective parameter has a specific dimension. In order to consider a logical relationship among these parameters, they should be made dimensionless. For this purpose, Eq. (5) has been used:
$${Z} = \frac{{{{{z} - \left[ {{z}_{\text{max} } + {z}_{\text{min} } } \right]} \mathord{\left/ {\vphantom {{{\text z} - \left[ {{\text z}_{\text{max} } + {\text z}_{\text{min} } } \right]} 2}} \right. \kern-0pt} 2}}}{{{{{z}_{\text{max} } - {z}_{\text{min} } } \mathord{\left/ {\vphantom {{{\text z}_{\text{max} } -{\text z}_{\text{min} } } 2}} \right. \kern-0pt} 2}}}$$(5)Here, Z denotes the independent dimensionless parameter, z represents the experimental data, zmax shows the maximum value of the independent experimental parameter, and zmin indicates the minimum value of the independent experimental parameter.
-
(4)
The fourth stage describes the interaction between the different experimental variables. This way, the model used in RSM is the second order model of the response surface g, which can be written as follows:
$$g = a_{0} + \sum\limits_{i = 1}^{n} {a_{i} x_{i} } + \sum\limits_{i = 1}^{n} {a_{ii} x_{i}^{2} } + \sum\limits_{ipj}^{{}} {a_{ij} x_{i} x_{j} } \pm \varepsilon$$(6)Here, \(a_{0} \text{,}a_{i} \text{,}a_{ii} \text{,}a_{ij}\) are constant terms, the coefficients of the linear parameters, and the coefficients of quadratic, denotes the coefficients of the interaction parameters respectively.
-
(5)
In the fifth stage, the mathematical model is unfamiliar, so one should check whether the model fits the experimental data well. For this purpose, methods such as residual analysis, scaling residual, prediction error sum of squares residual, and testing of the lack of fit can be applied. The comprehensive analytical ability of the model is commonly explained by the coefficient of determination (R2). Using analysis of variance (ANOVA), summarized in Table 3, R2 equation is as follows:
$$R^{2} = 1 - \frac{{{\text{SS}}_{\text{Error}} }}{{{\text{SS}}_{\text{Total}} }}$$(7)The closer the value of R2 is to one, the better is the regression. However, adding a variable to the model is the cause of the high value of R2. Hence, a different index is used to ensure the best of fitting, which is named adjusted R2. Adjusted R2 is a function of R2 that regulates the number of illustrative terms in a model. Unlike R2, the adjusted R2 rises as long as the new term improves the model more than would be expected otherwise. The adjusted R2 can be negative, and will always be less than or equal to R2. It is defined in Eq. (8).
$$R_{\text{adj}}^{2} = 1 - \frac{{\left( {1 - R^{2} } \right)\left( {n - 1} \right)}}{n - p - 1}$$(8)n is the number of input data, and p is the total number of variables in the model.
-
(6)
Draw three-dimensional graphics based on the obtained equations and determine the optimum points.
In this paper the independent variables are TR, TOS, and H2/CO ratio inlet gas and dependent variables are the selectivity of products from FTS that were obtained in the laboratory scale. Finally, mathematical relations will have been determined between them with the RSM.
4 Results and discussion
As mentioned above, the effect of TR, TOS, and H2/CO ratio inlet gas on the amount of selectivity production of FTS was investigated in this study using a mathematical model. The models involve three input variables—TR, TOS, and H2/CO—with five responses-selectivity of methane, light hydrocarbons, gasoline, diesel, and wax. The models were presented by RSM. In Table 2 the power of estimation of the RSM models are compared with experimental values. The regression parameters R2 and R 2adj are provided in Table 3.
4.1 Selectivity model for CH4
Figure 1 shows the changes in the selectivity of CH4 with TR, TOS, and H2/CO ratio. As be observed, with the concurrent decreased of TR and TOS, the low values of methane selectivity can be obtained at a constant H2/CO ratio. Furthermore, with decrease in H2/CO ratio and TR, the selectivity of CH4 can be lowered at a constant TOS. When the reduction temperature is a constant value, the selectivity of methane lessens with the increase of TOS and the decline of H2/CO ratio. In optimization, minimum methane selectivity is obtained as an aim. For this reason, the reaction conditions are considering as TOS = 404 h, TR = 242 °C, and H2/CO = 0.90 mol/mol. The model of methane selectivity (SC1) is as follows:
4.2 Selectivity model for light hydrocarbons
4.2.1 Selectivity model for light alkanes
Figure 2 represents the changes in the selectivity of light alkanes versus the effective parameters in Fischer–Tropsch reaction, namely TR, TOS, and H2/CO ratio inlet gas. When H2/CO ratio is constant, a better and higher selectivity of light alkanes can be obtained by increasing of the reduction temperature at high levels of TOS. In addition, when the time of stream is constant, increasing of the H2/CO ratio and TR simultaneously cause the increase of light alkanes selectivity. For optimization, the selectivity of light alkane maximizes, which can be obtained by the following conditions establishing: TOS = 422 h, TR = 279 °C, and H2/CO = 0.75 mol/mol. Equation (10) shows the selectivity model of light alkane (SC2–4):
4.2.2 Selectivity of light alkenes
Figure 3 expresses the changes in the selectivity of light alkenes in relation to TR, TOS, and H2/CO ratio inlet gas. To have a high selectivity of light alkenes, in constant value of H2/CO ratio, when the TR is low must avoided from high values of TOS. Similarly, when the time of stream is constant, for high level of light alkenes selectivity TR and H2/CO ratio value simultaneously must be maximized. The images indicate that if value of the H2/CO ratio and TOS are low, the value of selectivity of light alkenes will diminishes significantly. In optimization, the aim is to maximize of light alkenes selectivity. For this purpose, the conditions of the reaction must be considered as follows: TOS = 437 h, TR = 279 °C, and H2/CO = 0.75 mol/mol. The selectivity model for this product (SC =2–4 ) is obtained with Eq. (11).
4.3 The selectivity model of gasoline
Figures 4 and 5 represent the changes in the parameters of TR, TOS, and H2/CO ratio in relation to the variations in the gasoline selectivity. The degree of selectivity of paraffin and olefin in gasoline is increased with increases the TR at a constant value of TOS. In addition, at a constant value of TR, both factors TOS and H2/CO ratio must be kept at their maximum value in order to maintain the selectivity of gasoline at a high amount. However, when the H2/CO ratio is constant, the TOS and TR should be elevated to enhance the selectivity of paraffin and olefin present in gasoline. Eventually, in order to obtain the optimal conditions of paraffin and olefin present in gasoline respectively, the following values have been obtained: TOS = 491 h, TR = 244 °C, H2/CO = 0.77 mol/mol, TOS = 392 h, TR = 246 °C, and H2/CO = 0.78 mol/mol. Equations (12) and (13) represent the selectivity of paraffin (SC5–11) and olefin (SC =5–11 ) in gasoline respectively (Table 4).
4.4 The selectivity model of diesel
Figures 6 and 7 demonstrate the variations in the selectivity of diesel against TR, TOS, and H2/CO ratio inlet gas. At a constant value of H2/CO ratio, with the increase of TR and decrease of TOS, maximum selectivity of diesel can be expected. Furthermore, if value of H2/CO ratio and TR are brought to their maximum level, the value of selectivity of this product will be high. When the TR is constant, diesel selectivity would decline significantly if both TOS and H2/CO values are concurrently low. The optimization conditions for this product are obtained by considering the following: TOS = 437 h, TR = 251 °C, H2/CO = 0.77 mol/mol, TOS = 342 h, TR = 266 °C, and H2/CO = 0.75 mol/mol. The following equation has been developed for selectivity of diesel.
4.5 The selectivity model of wax
Figure 8 shows the wax selectivity changes due to the effects of TR, TOS, and H2/CO ratio inlet gas. Changes in H2/CO ratio and TOS against TR almost are the same. When the value of TR is low, increase of H2/CO ratio and TOS will increase the selectivity of wax. In high quantities of H2/CO, wax selectivity is improved by enhance the amount of TOS. The optimization objective is to obtain a high selectivity of wax, which can be attained with TOS = 283 h, TR = 280 °C and H2/CO = 0.81 mol/mol. The final mathematical model for selectivity of wax (\({\text{SC}}_{19}^{ + }\)) is Eq. (16):
5 Conclusions
Synthesis gas (H2, CO, CO2) obtain from forest wastes in high steam pressure and the free sulfur oil derivation are produced from them with the Fischer–Tropsch process. The determination of a selectivity model of every product is the great help for economic purposes, such that the best possible conditions for the economical production of favourite products are obtained with using these models. In this study, through the response surface methodology for the selectivity of five products—methane, light hydrocarbons, gasoline, diesel, and wax—in the Fischer–Tropsch reaction, eight selectivity models were stated in terms of the process conditions of the catalyst: TR, TOS, and H2/CO ratio inlet gas. These models indicated the TR change has a significant effect on the selectivity of products. So that, the TR decline causes the considerable decrease of methane and wax selectivity. To the production increase of light hydrocarbons, the amount of TR should have been increased. If objective is the minimization of all products selectivity and the maximization of selectivity of light olefin, the optimal operating conditions as TR = 258 °C, TOS = 375 h and H2/CO = 0.77 mol/mol will be considered.
Abbreviations
- ε :
-
The fitting error
- ψ :
-
The dependent variable or response
- a :
-
Constant terms
- g :
-
Unknown complex function of the independent variables
- i :
-
Counter
- j :
-
Counter
- n :
-
The number of input data, the sample size
- p :
-
The total number of explanatory variables in the model
- R 2 :
-
The coefficient of determination
- R 2adj :
-
Adjusted R2
- SSError :
-
The squared residuals with respect to the linear regression
- SSTotal :
-
The squared residuals with respect to the average value
- x :
-
The independent variables
- z :
-
The experimental data
- ANOVA:
-
Analysis of variance
- BET:
-
Brunauer–Emmett–Teller
- FTS:
-
Fischer–Tropsch synthesis
- RSM:
-
Response surface methodology
- STSR:
-
Stirred tank series reactor
- TOS:
-
Time on stream (h)
- TR:
-
Temperature reduction (°C)
References
Abelló S, Montané D (2011) Exploring iron-based multifunctional catalysts for Fischer–Tropsch synthesis: a review. ChemSusChem 4:1538–1556
Aitken ML, Loughlin DH, Dodder RS, Yelverton WH (2016) Economic and environmental evaluation of coal-and-biomass-to-liquids-and-electricity plants equipped with carbon capture and storage. Clean Technol Environ Policy 18:573–581
Ayodele BV, Khan MR, Nooruddin SS, Cheng CK (2017) Modelling and optimization of syngas production by methane dry reforming over samarium oxide supported cobalt catalyst: response surface methodology and artificial neural networks approach. Clean Technol Environ Policy 19:1181–1193
Bukur DB, Sivaraj C (2002) Supported iron catalysts for slurry phase Fischer–Tropsch synthesis. Appl Catal A 231:201–214
Bukur DB, Lang X, Akgerman A, Feng Z (1997) Effect of process conditions on olefin selectivity during conventional and supercritical Fischer–Tropsch synthesis. Ind Eng Chem Res 36:2580–2587
Bukur DB, Lang X, Ding Y (1999) Pretreatment effect studies with a precipitated iron Fischer–Tropsch catalyst in a slurry reactor. Appl Catal A 186:255–275
Bukur DB, Lang X, Nowicki L (2005) Comparative study of an iron Fischer–Tropsch catalyst performance in stirred tank slurry and fixed-bed reactors. Ind Eng Chem Res 44:6038–6044
Domínguez-García S, Gutiérrez-Antonio C, De Lira-Flores JA, Ponce-Ortega JM (2017) Optimal planning for the supply chain of biofuels for aviation in Mexico. Clean Technol Environ Policy 19:1387–1402
Duvenhage D, Coville N (2002) Fe:Co/TiO2 bimetallic catalysts for the Fischer–Tropsch reaction: part 2. The effect of calcination and reduction temperature. Appl Catal A 233:63–75
González-Delgado Á-D, Kafarov V, El-Halwagi M (2015) Development of a topology of microalgae-based biorefinery: process synthesis and optimization using a combined forward-backward screening and superstructure approach. Clean Technol Environ Policy 17:2213–2228
Hao Q-L, Liu F-X, Wang H, Chang J, Zhang C-H, Bai L, Xiang H-W, Li Y-W, Yi F, Xu B-F (2007) Effect of reduction temperature on a spray-dried iron-based catalyst for slurry Fischer–Tropsch synthesis. J Mol Catal A Chem 261:104–111
Landau MV, Vidruk R, Herskowitz M (2014) Sustainable production of bioparaffins in a crude oil refinery. ChemSusChem 7:785–794
Li J, Cheng X, Zhang C, Yang Y, Li Y (2015) Effects of alkali on iron-based catalysts for Fischer–Tropsch synthesis: CO chemisorptions study. J Mol Catal A Chem 396:174–180
Liu Y, Ersen O, Meny C, Luck F, Pham-Huu C (2014) Fischer–Tropsch reaction on a thermally conductive and reusable silicon carbide support. ChemSusChem 7:1218–1239
Lueking AD, Cole MW (2017) Energy and mass balances related to climate change and remediation. Sci Total Environ 590:416–429
Ma W, Jacobs G, Graham UM, Davis BH (2014) Fischer–Tropsch synthesis: effect of K loading on the water–gas shift reaction and liquid hydrocarbon formation rate over precipitated iron catalysts. Top Catal 57:561–571
Ma W, Jacobs G, Sparks DE, Shafer WD, Hamdeh HH, Hopps SD, Pendyala VRR, Hu Y, Xiao Q, Davis BH (2016) Effect of H2S in syngas on the Fischer–Tropsch synthesis performance of a precipitated iron catalyst. Appl Catal A 513:127–137
Ngantsoue-Hoc W, Zhang Y, O’Brien RJ, Luo M, Davis BH (2002) Fischer–Tropsch synthesis: activity and selectivity for group I alkali promoted iron-based catalysts. Appl Catal A 236:77–89
Noureldin MM, Bao B, Elbashir NO, El-Halwagi MM (2014) Benchmarking, insights, and potential for improvement of Fischer–Tropsch-based biomass-to-liquid technology. Clean Technol Environ Policy 16:37–44
Rafati M, Wang L, Shahbazi A (2015) Effect of silica and alumina promoters on co-precipitated Fe–Cu–K based catalysts for the enhancement of CO2 utilization during Fischer–Tropsch synthesis. J CO2 Util 12:34–42
Silva DO, Scholten JD, Gelesky MA, Teixeira SR, Dos Santos ACB, Souza-Aguiar EF, Dupont J (2008) Catalytic gas-to-liquid processing using cobalt nanoparticles dispersed in imidazolium ionic liquids. ChemSusChem 1:291–294
Suo H, Wang S, Zhang C, Xu J, Wu B, Yang Y, Xiang H, Li Y-W (2012) Chemical and structural effects of silica in iron-based Fischer–Tropsch synthesis catalysts. J Catal 286:111–123
Todic B, Nowicki L, Nikacevic N, Bukur DB (2016) Fischer–Tropsch synthesis product selectivity over an industrial iron-based catalyst: effect of process conditions. Catal Today 261:28–39
Vengsarkar PS, Xu R, Roberts CB (2015) Deposition of iron oxide nanoparticles onto an oxidic support using a novel gas–expanded liquid process to produce functional Fischer–Tropsch synthesis catalysts. Ind Eng Chem Res 54:11814–11824
Wan H-J, Wu B-S, Zhang C-H, Xiang H-W, Li Y-W, Xu B-F, Yi F (2007) Study on Fe–Al2O3 interaction over precipitated iron catalyst for Fischer–Tropsch synthesis. Catal Commun 8:1538–1545
Wan H, Wu B, Zhang C, Xiang H, Li Y (2008) Promotional effects of Cu and K on precipitated iron-based catalysts for Fischer–Tropsch synthesis. J Mol Catal A Chem 283:33–42
Weckhuysen BM (2013) Recalcitrance of nature: chemocatalysis for the production of biomass-based building blocks. ChemSusChem 6:1559–1563
Xinling L, Zhen H (2009) Emission reduction potential of using gas-to-liquid and dimethyl ether fuels on a turbocharged diesel engine. Sci Total Environ 407:2234–2244
Zhang Q, Kang J, Wang Y (2010) Development of novel catalysts for Fischer–Tropsch synthesis: tuning the product selectivity. ChemCatChem 2:1030–1058
Zhang Q, Cheng K, Kang J, Deng W, Wang Y (2014) Fischer–Tropsch catalysts for the production of hydrocarbon fuels with high selectivity. ChemSusChem 7:1251–1264
Acknowledgements
The authors gratefully acknowledge Iran’s Ministry of Science and Research and University of Sistan and Baluchestan for helping and financial supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Atashi, H., Veiskarami, S. Green fuel from coal via Fischer–Tropsch process: scenario of optimal condition of process and modelling. Int J Coal Sci Technol 5, 230–243 (2018). https://doi.org/10.1007/s40789-018-0204-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-018-0204-7