Highlights
-
Polyimides (PIs) as coatings, separators, binders, solid-state electrolytes, and active storage materials help toward safe, high-performance, and long-life lithium-ion batteries (LIBs).
-
Strategies to design and utilize PI materials have been discussed, and the future development trends of PIs in LIBs are outlooked.
Abstract
Lithium-ion batteries (LIBs) have helped revolutionize the modern world and are now advancing the alternative energy field. Several technical challenges are associated with LIBs, such as increasing their energy density, improving their safety, and prolonging their lifespan. Pressed by these issues, researchers are striving to find effective solutions and new materials for next-generation LIBs. Polymers play a more and more important role in satisfying the ever-increasing requirements for LIBs. Polyimides (PIs), a special functional polymer, possess unparalleled advantages, such as excellent mechanical strength, extremely high thermal stability, and excellent chemical inertness; they are a promising material for LIBs. Herein, we discuss the current applications of PIs in LIBs, including coatings, separators, binders, solid-state polymer electrolytes, and active storage materials, to improve high-voltage performance, safety, cyclability, flexibility, and sustainability. Existing technical challenges are described, and strategies for solving current issues are proposed. Finally, potential directions for implementing PIs in LIBs are outlined.

Similar content being viewed by others
1 Introduction
The ongoing energy crisis and environmental issues attributed to traditional energy sources have aroused extensive attention [1, 2]. New energy sources in place of traditional energy sources have become an inevitably developing trend. Lithium-ion batteries (LIBs), as one of the most attractive energy sources, have been commercialized for decades [3,4,5,6,7]. However, several technological challenges such as increasing energy density, improving safety, and extending longevity still urgently need to be overcome. Overview of the rapid development of LIBs in the past 30 years, the critical issues solved and significant technical innovations all benefit from the advancements of state-of-the-art materials [8, 9]. The era of new materials has arrived.
LIBs are composed of the cathode, anode, separator, electrolyte, and coin shells involving various constituent materials incorporation of organic materials, inorganic materials, and metals. Particularly, polymers, as one kind of organic material, are not extensively used in LIBs, besides the separator and binder. However, with the continued improvement of researchers’ cognitions, more polymeric components are now being considered for LIBs [10, 11], including different functional polymer coatings, the current popular solid-state polymer electrolytes, new polymer active storage materials, etc. Nowadays, two dilemmas of polymers applied in LIBs should be dealt with. One is that several technique and usage problems faced by traditional polymer components limit their practical applications, which should be replaced by new-style polymer materials. For example, traditional polyolefin separators cannot withstand high temperatures, which replaced by high-performance separators is encouraged. The other one is using new polymer components to boost LIBs. One compelling case is employing functional polymer coatings to protect key components from deteriorations of high voltage or interfacial side reactions. Additionally, new styles of solid-state polymer electrolytes and polymer active storage materials are also developing trends. In a word, polymer materials are playing more and more important roles in LIBs.
LIBs propose very high-performance requirements for polymer materials, including excellent chemical resistance, thermal stability, high-voltage tolerance, and even high mechanical strength, while polyimides (PIs) are standing out among many polymers. PIs can be used as coatings [12], binders [13], separators [14], solid-state electrolytes [15], active storage materials [16], etc. Though the applications of PIs are diverse, they are still confined to the laboratory and far away from commercialization. PIs are formed from anhydride and amine polycondensations [17]. PIs have been in development since their introduction in 1955. There are two methods for synthesizing PIs: One is the hydrothermal method, also called the one-step method, and the other is the two-step method, which includes thermal imine and chemical imine methods [18]. The hydrothermal method involves direct polymerization in a high boiling point solvent. The high temperatures and pressures involved in the procedure can be a safety concern. The chemical imine method refers to PI precursors (polyamic acid, PAA) dehydrated and cyclized with the aid of a catalyst. Compared with these two methods, the thermal imine method involves PAA cyclization at high temperatures without introducing other substances, confirming to be a more economical and convenient way. PIs can be categorized into aliphatic and aromatic. Generally, aliphatic PIs are flexible and soluble, mainly used as coatings or binders. In contrast, aromatic PIs are rigid and insoluble, mainly used as membranes or solid powders. Table 1 lists the important physical and chemical properties of different PI applications in LIBs. PIs are promising in LIBs. PIs can be coated onto active material surfaces to keep the cathode and electrolyte interface stable at high-voltage conditions, increasing the cyclability of the cell. PIs can also be used as separators to improve safety when operating at high temperatures. To prolong the battery life, researchers can employ PI binders to enhance the electrode structural integrity. PIs can also be employed in solid-state lithium batteries. Furthermore, PIs can replace traditional energy storage materials to lower the cost and environmental pollution. Figure 1 outlines the requirements of each LIB component that can be satisfied by incorporating PIs into LIBs.
Schematic showing how polyimides (PIs) are promising polymer materials for LIBs. As coatings, PIs can stabilize the interface of the cathode and electrolyte. PI separators can enhance thermal stability, while PI binders can maintain the structural integrity of electrodes. PIs can increase the energy density of LIBs if employed as solid-state electrolyte support. PIs can replace traditional inorganic active storage materials. All these promising PI applications help toward safe, high-performance, and long-life LIBs
From PI materials to LIBs, much effort should be devoted to solving various issues encountered. Herein, we summarize the important issues involving PIs in LIBs. We describe the essential features for each issue encountered when employing PIs. Moreover, we address several critical technology challenges and strategies for using PIs in LIBs. Finally, future development directions for LIBs with PIs are outlined.
2 PIs in Different LIB Components
2.1 Improving High-Voltage Performance: PI Coatings on Cathode Materials
Energy storage devices with high energy and power densities for portable electric devices, electric vehicles, and grid energy storage are being investigated intensively [19]. To increase the energy density of LIBs, researchers have two strategies: increase the specific capacity or increase the operating voltage according to Eq. 1:
where \(E\) represents the energy density, \(C_{0}\) is the theoretical specific capacity, and \(V\) is the average operating voltage. For cathode materials, much effort has been devoted to finding materials that operate below 4.35 V but exhibit high specific capacity, i.e., Ni-rich LiMO2 (M = Co, Mn, Ni) [20,21,22,23]. However, the practical specific capacity of these materials is nearing the limit of ~ 200 mAh g−1. Therefore, researchers began to increase the specific capacity by elevating the operating voltage.
When LIBs are at high-voltage conditions, the delithiated cathode and liquid electrolyte promote violent interfacial side reactions, which is the most significant challenge researchers face. Surface coating is an effective technique that reduces interfacial side reactions, thereby enhancing electrochemical performance. To date, many metal compounds, such as Al2O3 [24,25,26,27], ZrO2 [28], MoO3 [29], TiO2 [30], AlF3 [31], and Li3PO4 [32], have been used to coat active materials. Because these metal compounds facilitate Li-ion conductivity and decrease the violent side reactions, irreversible lithium loss is reduced and cycling performance is increased. However, metal oxides face several technical issues, including discontinuity, brittleness, and delamination from the surface of cathode materials. Therefore, selecting polymers with tunable molecular structures and high adhesion as coatings can be an effective strategy.
Among the polymers, PIs with excellent performances such as good elasticity, thermal stability, and electrolyte wettability can be used as ideal coating materials [33, 34]. Especially as coatings, PIs should be insoluble in electrolytes as well as possess good electrolyte wettability. This performance requirement is important for enhancing the electrochemical performance. The synthesis of PI-coated cathode materials is the focus of this section, and the coating process is shown in Fig. 2a. Herein, the thermal imine method is used to realize the coating. First, pyromellitic dianhydride (PMDA) and 4,4′-oxydianiline (ODA) are polymerized to obtain PAA at low temperatures. Then, a certain concentration of PAA solution is mixed with cathode active materials to coat the PAA onto the surface of active material particles. Thereafter, the PAA-coated cathode materials are thermally imidized through a step-by-step heating method to convert PAA to PI coatings [35]. Finally, active material particles with PI nano-coatings are obtained.
a Schematic of PI coating onto an LNMCO cathode material. b TEM image of PI-coated LiNi1/3Mn1/3Co1/3O2 (PI@NCM333). c Electrochemical performances of LNMCO, PI@LNMCO-300, and PI@LNMCO-450, including initial efficiency, cycling, and rate capability (300 and 450 represent thermal imidization temperatures). d Schematic of the mechanism for enhanced electrochemical performances in PI-coated NCM333
Transmission electron microscopy (TEM) is the most straightforward way for evaluating PIs successfully coating onto the surface of cathode particles. Figure 2b is a TEM image of PI-coated LiNi1/3Co1/3Mn1/3O2 (NCM333). The coating was continuous and uniform (~ 10 nm thick) [33]. The electrochemical performance of the cell was mainly affected by this PI layer, as shown in Fig. 2c. As seen in this figure, the cyclability of PI-LNMCO (Li1.2Ni0.13Mn0.54Co0.13O2) is better than that of LNMCO, especially at high thermal imine temperatures. Zhang et al. [35] found that high thermal imidization temperatures facilitate strong interactions between PI coatings and cathode particle surfaces, prohibiting the solvation of transition metal ions and enhancing the electrochemical performance. Figure 2d illustrates the PI-coating mechanism [33]. PI effectively blocks the electrolyte, hindering interfacial side reactions. Meanwhile, Li-ion conductivity in PIs is good [36]. The cathodes with PI coatings exhibit a lower polarization and less irreversible Li-ion loss than uncoated cathodes, resulting in a good cycling lifetime. Additionally, N has special interactions with Mn4+, further increasing the rate capability. Currently, the discussion focus is the stability of PI coatings at high voltage. Meanwhile, our group has devoted much work to verifying this question. Our following work concludes that PI coatings are stable at high voltages up to 4.6 V without structural changes. PI coatings play the physical barrier role in retarding the interfacial side reactions. The optimal PI-coating content is about 4wt% (PAA added in the preparation process). As a consequence, PI coatings provide an effective way to improve electrochemical performances at high-voltage conditions.
2.2 Improving Battery Safety: PI Separators
The LIB separator is a porous and insulating membrane located between the cathode and anode, not only providing a pathway for Li-ion conduction but also playing the role of avoiding internal short circuits (ISCs) [37, 38]. Higher demands such as the proper pore size, uniform pore distribution, low pore tortuosity, low heat shrinkage, excellent mechanical performance, and good electrolyte wettability should have for separators [39]. Therefore, the materials chosen and processing technologies for the preparation of good-performance separators are strict.
Commercial separators are made of polyethylene (PE) or polypropylene (PP) owing to their high chemical stability and acceptable mechanical strength. Currently, the processing technologies for polyolefin separators are very mature. The thickness of polyolefin separators can be reduced to less than 15 μm without sacrificing their mechanical property, and the electrochemical performance can be obtained stably at room temperatures. However, two shortcomings of these separators need to be addressed [40]. First, the wettability of polyolefin separators is insufficient in accommodating any electrolyte, resulting in limited ionic conductivity owing to their hydrophobic nature. Second, the high thermal shrinkage and low melting temperature of polyolefin separators easily induce ISCs when operating at high temperatures, leading to thermal runaway. Compared with polyolefin separators, PI membranes exhibit a higher tensile strength of more than 10 Mpa, higher thermal stability up to 500 °C with little shrinkage, and good electrolyte uptake of larger than 100%. Shi et al. [41] have found that the above performances of PI separators are all better than those of PE separators. He et al. [42] employed different structural monomers to synthesize diverse PI separators, and all performance metrics are higher than those of a PP separator. These findings demonstrate that PI separators are preferable separator candidates.
PI separators are not yet commercialized due to their complex processing technology and expensive cost. To date, the preparation methods for PI separators are still confined to the laboratory, including template, phase separation, and electrospinning methods. Figure 3a shows a schematic of the template method reported by Lin et al. [43]. Typically, the template method employs inorganic salts as templates to make pores. In their work, LiBr and SiO2 were mixed into a PAA solution and doctor blade to produce the membrane. Then, the PAA membrane was thermally imidized to convert it into a PI membrane. Finally, a nanoporous PI membrane was obtained by washing LiBr away with water. The main advantage of this method is that it maintains the intact pore structure. Moreover, the added SiO2 enhances electrolyte wettability even further and provides good thermal stability and mechanical properties to the separator, which could widen the service temperature and prohibit Li dendrites effectively. Li et al. [44] reported the phase separation method to fabricate PI separators. As shown schematically in Fig. 3b, dibutyl-phthalate and glycerin are added to the PAA solution, which is then blade onto a glass plate to obtain the PAA membrane. This PAA membrane is further immersed in an ethanol coagulation bath at 40 °C several times to remove the solvent and additives for making holes. Finally, the same method of thermal imidization is adopted to convert the PAA membrane into a PI membrane. The cell assembled with this PI separator exhibited good electrochemical performances at 140 °C, whereas the cell with a PE separator failed. The reason for the good performance of the cell at high temperatures is attributed to the PI separator maintaining good thermal stability without observable shrinkage at high temperatures. Similarly, PI separators prepared by the modified method have been further explored by Song and Zhou et al. [45, 46] to boost the safety of LIBs. Compared with the above methods, the electrospinning method is simpler and more versatile for membrane preparation. Electrospinning is one of the most promising methods to realize PI separator commercialization. Li et al. [47] employed the solution blow spinning for fabricating PI separators. The technique includes three steps: ①synthesizing PAA, ② electrospinning, and ③ thermal imidization. The preparation process is shown in Fig. 3c. As the PI separator made by the electrospinning method possesses a conductive pore structure, it further enhances the ionic conductivity. While the size of the hole is large, the formed Li dendrites easily penetrate the separator and lead to the ISCs. Therefore, many references have reported the modified electrospinning PI separators.
Pore structure modifications include coating with polymers or inorganic ceramic particles. A polymer coating could reduce pore size to prevent Li dendrites and improve mechanical properties and thermal stability. Besides, inorganic ceramic particle coatings are also beneficial for enhancing electrochemical performances. Currently, much work has been done for PI separators coated with inorganic ceramic materials combined with the electrospinning method [48,49,50]. Figure 4a illustrates a PI nanofiber separator coated with SiO2 particles using the in situ nanoencapsulation hydrolysis method, as reported by Wu et al. [48]. SEM images clearly show that SiO2 coatings are uniformly wrapped onto the surfaces of PI nanofibers. The static contact angle of the carbonate-based electrolyte on the PI/SiO2 separator was 6.8° as shown in Fig. 4b, lower than that on Celgard and pure PI separators, indicating good electrolyte wettability of the PI/SiO2 separator. Qiao et al. [50] fabricated another PI separator reinforced with intercalated organic montmorillonite (OMMT) via solution blow spinning. In Fig. 4c, the cell with the as-prepared PI/OMMT composite separator exhibits better cycling performance (a discharge specific capacity of 131 mAh g−1 after 100 cycles at C) and rate capability (124 mAh g−1 at 2 C) than the one with Celgard-2500 separator (107 mAh g−1 after 100 cycles at 1 C and 107 mAh g−1 at 2 C). This is due to that the electrolyte wettability of the PI/OMMT composite separator is enhanced, which forms the complete Li-ion conductive route and induces higher Li-ion conductivity, resulting in the enhancement of electrochemical performances. The ceramic-coated PI separators also have good mechanical flexibility and flame-retardant properties. Figure 4d shows that PI separators are rollable, foldable, and twistable, guaranteeing good use reliability [49]. Figure 4e shows the comparison of the combustion performances of Celgard-2400, pure PI, and PI/ZrO2 separators. The PI/ZrO2 separator exhibits the best flame-retardant property, suggesting that incorporating inorganic ceramic particles is beneficial in improving the safety of LIBs [49].
a SEM images of PI/SiO2 nanofibers. b Static contact angles of Celgard-2400, pure PI, and PI/SiO2 separators (43°, 15°, and 6.8°, respectively). c Cycling and rate capability performances of Celgard-2500, PI, and PI/OMMT separators. d Images of the PI/SiO2 separator demonstrating its flexibility. e Flame retardancy of Celgard-2400, pure PI, and PI/SiO2 separators
2.3 Improving Cyclability of Structurally Integral Anodes: PI Binders
Graphite dominates the anode market for many years owing to its long cycle life, abundance, and low cost. However, the graphite anode material possesses a relatively lower energy density (375 mAh g−1). Therefore, developing alternative anode materials is essential. Silicon is considered to be a promising anode material for next-generation LIBs owing to its abundance and high theoretical specific capacity (4,200 mAh g−1). The main challenge in silicon anodes is their extensive volume change (up to 300%) during lithium insertion and extraction, which often leads to the pulverization of active alloy particles and poor cyclability. Nano-silicon [51, 52] and Si/C composite [53,54,55] can mitigate volume change in silicon. Using suitable binders [56,57,58,59,60,61] could also prevent silicon particle pulverization. Although the amount of binders in electrodes is little, they influence the performance of silicon-based anodes significantly.
Various polymer binders, such as the mussel-inspired binder [62,63,64], polyacrylic acid [58, 65], polysaccharides [66, 67], polyacrylonitrile [68], polyimides [69,70,71], self-healing polymer binders [61, 72], and conductive binders [73, 74], have been developed. Among these, PI binders with superior adhering properties, good mechanical strength, remarkable thermal stability, and outstanding electrolyte affinity are attracting wide attention. Much work reports PI binders from the perspective of molecular structure design, aiming at ameliorating different properties. To increase the Li-ion conductivity, Yao et al. [75] introduced ether functional group abundant polyethylene glycol (PEG) segments to PI structure. Compared with the carboxymethyl cellulose (CMC) binder, the discharge capacity of the silicon anode using this PI binder is as high as 2,989.7 g−1 and with few microcracks generated after cycling shown in Fig. 5a. To depress the pulverization of silicon anode, the soft and rigid copolymerized PI binder was designed [76]. The soft siloxane components possess excellent elasticity to accommodate volume changes. The rigid blocks provide a high modulus to withstand the mechanical stress generated in the silicon anode. The structural diagram of poly(siloxane imide) (PSI) binder is shown in Fig. 5b. To increase the adhesive property, PI binders with stronger adhesive functional groups were synthesized. Choi et al. [77] used highly adhesive and thermally stable co-polyimide (P84) as the silicon anode binder. In Fig. 5c, P84-based electrode was peeled off from the Cu current collector with little residues, while the surface of the Cu current collector was smooth after the PVDF-based electrode was peeled off, suggesting better adhesion with P84 than with PVDF. Besides, high adhesive capability of PI binders could keep the electrode with structural integrity, leading to the good cycling life and rate capability. Xu et al. [78] designed a kind of PI binder with the functional groups of carboxyls tethered laterally shown in Fig. 5d. Due to the interaction between carboxyls and the surface oxide layer of silicon particles, it prohibits the regenerations of solid electrolyte interface (SEI), enhancing the electrochemical performance. To sum up, the reasonable structural design of PI binders endows silicon anodes with better structural integrity and longer cycling lifespan, which lays a good foundation for the commercialization of silicon anodes.
a PI-200 synthesis by polymerizing polyethylene glycol (PEG), trimellitic anhydride chloride (TMAC), and 4,4′-methylenedianiline (MDA). Cycling performances, SEM images after cycling, and schematics of lithiation/delithiation comparing PI-200 and CMC binders are also shown. b Alternative PI binder design combining soft and rigid segments. c P84 vs. PVDF binder in silicon anodes. Images from peeling experiments reveal adhesion effects. d The synthesis process of PI-COOH binder, cycling and rate capability, and SEI formations
As PI materials are stable at high voltage, it concludes that PI binders are also stable at high voltage. Combining the merits of good structural integrity and high-voltage resistance, PI binders can be applied in cathodes. Similarly, the design of PI binders for cathodes is purposive. Pham et al. [79] designed a block copolymerized PI binder containing –CF3 and –COOH. Due to the –COOH, this PI binder will form strong interactions with LiNi0.8Co0.1Mn0.1O2 (NCM811), whose structure is shown in Fig. 6a. Due to the –CF3, it endows the PI binder with good thermal stability and chemical inertness. The structure of the electrode piece is shown in Fig. 6b, and the thickness of the binder layer is about 3 nm shown in Fig. 6c. Qi et al. [80] also designed two kinds of PI cathode binders, namely PI(BBP) and PI(OBO). These two PI binders were, respectively, mixed with active materials LiNi0.8Co0.1Mn0.1O2 (NCM811) and conducting materials super-P in the ratio of 8:1:1 to obtain cathode sheet, whose cross-sectional SEM image and the corresponding schematic diagram are shown in Fig. 6d. The CR2032 coin cells assembled with these two kinds of cathodes show good rate capability and cycling performance in Fig. 6e. No matter PI binders are used for cathodes or silicon anodes, the electrode structural integrity can be assured, leading to excellent electrochemical performances.
2.4 Application in All-Solid-State Lithium-Ion Batteries: Solid PI-based Electrolytes
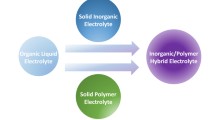
To realize all-solid-state (ASS) lithium-ion batteries (LIBs) with higher safety and higher energy density, solid-state electrolytes (SSEs) are pivotal as they play a significant role in determining comprehensive electrochemical performance [81, 82]. SSEs should be highly ionically conductive, mechanically strong, chemically/electrochemically stable, nonvolatile, and non-flammable. SSEs can be classified into three types: inorganic (ceramic/glass) solid electrolytes (ISEs), solid polymer electrolytes (SPEs), and composite solid-state electrolytes (CSEs) [83]. Though ISEs possess excellent Li-ion conductivity, the intrinsic brittleness and easy pulverization of ISEs affect their use reliability. SPEs present continuous and flexible features, while SPEs face a big challenge in the improvement of Li-ion conductivity. Combining both advantages of ISEs and SPEs, CSEs show great development potential in SSEs.
For preparing SPEs even CSEs, choosing a suitable polymer matrix is the first consideration. Poly(ethylene oxide) (PEO) has become the most widely used electrolyte material owing to its high solubility toward lithium salts and relatively high Li-ion conductivity. However, PEO-style SSEs do not prohibit Li dendrites and are not stable at high temperatures. PIs possess excellent mechanical strength, good electrolyte wettability [84], and outstanding thermal stability, suggesting that they can be a good candidate for the SSE matrix. Generally, PI SSEs could be categorized as solid PI and solid PI-based electrolytes. Solid PI electrolytes are synthesized by introducing PEO molecular segments to PI main chains, while the solid PI-based electrolytes are synthesized by introducing PEO electrolytes to a PI porous membrane. Currently, only a few studies have employed PI directly as an electrolyte [85]. Much work has been reported on solid PI-based electrolytes. Furthermore, solid PI-based electrolytes are divided into solid PI-based SPEs and solid PI-based CSEs.
For solid PI-based SPEs, Wan et al. [86] fabricated a kind of ultrathin and flexible solid PI-based electrolytes, as shown in Fig. 7a(i). Aligned channels were made on the thin Kapton PI film by using the track-etching method. Then PEO/lithium bis-(trifluoromethanesulfonyl)imide (LiTFSI)/acetonitrile solution was dropped and spun on this porous PI membrane to infiltrate the pores. SEM images for the cross-sections of the PI SPE before and after cycling are shown in Fig. 7a(ii). The thickness of the PI SPE is ~ 8 μm. The electrode surface is smooth even after cycling, demonstrating Li dendrites were effectively prohibited by the PI SPE, as shown in Fig. 7a(iii). Additionally, the PI SPE-assembled pouch cell can still operate under folding, twisting, and unfolding shown in Fig. 7b(i, ii) and the pouch cell can still light an LED bulb after the nail and cutting tests shown in Fig. 7b(iii, iv). To further increase the safety, Cui et al. [87] designed a flame-retardant PI SPE with a fire-retardant additive, decabromodiphenyl ethane (DBDPE) cast on the PI matrix, whose structure is shown schematically in Fig. 7c. In Fig. 7d(i), the PE, PEO/LiTFSI, and PI/DBDPE membranes exhibit different thermal behaviors under high-temperature conditions. Due to the different melting points of the PE, PEO, and PI matrix, PE shrank, PEO melted, while PI can withstand high temperatures and maintain its original morphology. In extreme cases shown in Fig. 7d(ii, iii), the PE membrane-assembled pouch cell can no longer function when burned. The LED bulb was off after 18 s. Under the same conditions, the pouch cell assembled with the PEO/LiTFSI SPE no longer functions after 24 s. However, the pouch cell assembled with the PI/DBDPE/PEO/LiTFSI SPE still functions for more than 24 s. The excellent work shows that the PI SPE has a great commercial prospect for safety.
a Vertical porous nanochannels made using the track-etching method on a PI film (i), obtaining ultrathin, lightweight, and flexible PI solid polymer electrolytes (ii). A uniform Li deposition layer was observed by SEM after cycling (iii). b Abuse tests for PI/PEO/LiTFSI-based LIBs: (i) folding performance; (ii) button batter lighting an LED bulb; (iii) flexible Li/PI/PEO/LiTFSI/LFP pouch cell lighting an LED bulb; and (iv, v) nail and cutting tests. c Flame-retardant PI SPE by adding the fire-retardant DBDPE. d Thermal abuse tests: (i) Photographs of the PE separator, PEO/LiTFSI, and PI/DBDPE film before and after exposure to thermal shock (150 °C, 0.5 h); (ii) schematic illustration of the thermal abuse test of the pouch cell battery; and (iii) flame abuse test of batteries with EC/DEC/PE, PEO/LiTFSI, and PI/DBDPE/PEO/LiTFSI as electrolytes
For solid PI-based CSEs, Hu et al. [88] used the blading method to prepare a PI CSE. The porous PI film was used as the host, and LLZTO nanoparticles with PVDF/LiTFSI were used as electrolyte fillers. The structures of PVDF SPE, LLZTO/PVDF CSE, and PI-LLZTO/PVDF CSE are shown in Fig. 8a(i-iii), where the tensile strength of the PI-LLZTO/PVDF CSE membrane is the best shown in Fig. 8b. The Li-ion plating/stripping mechanism is schematically illustrated in Fig. 8c(i, ii). The result demonstrates that PI CSE exhibits better mechanical strength to resist Li dendrites and forms a more uniform Li deposition layer than the non-PI-based CSEs. Gai et al. [89] prepared an asymmetric PI CSE using the casting method with Li1.3Al0.3Ti1.7(PO4)3, poly(vinylidene fluoride-hexafluoropropylene) [P(VDF-HFP)], and succinonitrile cast on the PI film. The SEM image of the cross section of the PI-PVDF-HFP CSE is exhibited in Fig. 8d. The porous structure is beneficial to Li-ion conductivity. The Li||Li+ coin cell assembled with the PI-PVDF-HFP CSE presents better cycling performance than PVDF-HFP CSE, demonstrating that PI materials are good solid-state electrolyte hosts. The PI-PVDF-HFP CSE is thermally stable at 150 °C and is flame retardant, which is described in Fig. 8e. Notably, the suitable processing methods combined with the outstanding properties of PIs make solid PI-based electrolytes promising SSE candidates in all-solid-state LIBs.
a Schematic diagrams of Li-dendrite layers of PVDF SPE (i), LLZTO/PVDF CSE (ii), and PI-LLZTO/PVDF CSE (iii). b Mechanical property characterization (inset is the physical morphology). c Schematic illustrations of Li-plating/stripping behavior in Li/LLZTO/PVDF CSE/Li (i) and Li/PI-LLZTO/PVDF CSE/Li (ii). d SEM image of PI CSE and cycling stability of the symmetric cells assembled with PVDF-HFP and PI-PVDF-HFP CSEs. e Thermal stability and flame retardancy of PP and PI films
Electrospinning technology can also be used to prepare the scaffold of solid-state electrolytes. Shen et al. [90] take the electrospinning technology to make core–shell structured gel polymer electrolytes, whose preparation process is shown in Fig. 9a. In detail, the electrolyte solution PVDF-HFP/PMASLi and PI solution were injected into the coaxial nozzle, spun onto the collector wrapped with Al foil, then took it off, and carried on the post-treatment, obtaining the PI-based solid-state electrolytes. Another preparation of PI-based solid-state electrolytes [91] is to make a PI electrospinning membrane, then blend it with PEO/LiTFSI electrolyte solution, and obtain the PI-base solid-state electrolytes, as shown in Fig. 9b. Due to its high mechanical strength, good thermal stability, and excellent (electro)chemical inertness, the cell assembled as Li|PEO/LiTFSI/PI SPE|Li showed better cycling and rate performance than the common cell of Li|PEO/LiTFSI SPE|Li. It follows that the preparation methods of PI separators can be effectively used as reference to prepare PI-based electrolytes.
a Preparation process of the core–shell gel polymer electrolyte by the coaxial spinning technology. b (i) Schematic sketch of PEO/LiTFSI/PI fibers CSPE, (ii) cycling performance of Li|PEO/LiTFSI/PI SPE|Li battery at 0.1 mA cm−2, and (iii) rate performance of Li|PEO/LiTFSI/PI SPE|Li battery at different current densities
2.5 Next-generation of Organic Electrodes: PI Active Storage Materials
In LIBs, active storage materials play a crucial role: lithium-ion storage. Based on the different lithium-ion storage mechanisms of cathodes and anodes, active storage materials can be classified into two kinds: cathode and anode. Cathode active storage materials are mainly lithium-contained transition metal oxides, while anode active storage materials are commonly using graphite. Conventional cathode active storage materials are price costive and resource-scarce, limiting their large-scale energy storage applications in long term. Furthermore, the transition metal elements of cathode active storage materials are toxic and environmentally polluting, which recycles difficult, requires tedious procedures, and consumes a lot of energy. Compared with cathode active storage materials, the reserves of graphite are abundant. But the flammability and the goal of reducing carbon emissions force us to search for other alternative materials, such as silicon. Therefore, developing renewable and sustainable materials as active storage materials for LIBs is critical.
The root of lithium-ion storage for organic materials is based on the reversible lithium-ion combination reactions. Presently, many different organic functional groups such as organosulfur compounds [92, 93], nitroxyl radical-bearing compounds [94, 95], conjugated carbonyl-containing compounds [96], imine-functionalized compounds [97, 98], azo-functionalized compounds [99, 100], and newly appeared sulfonamides compounds [101,102,103] have been used as cathode materials in LIBs. Nevertheless, several small organic molecules can dissolve into the electrolyte, further affecting cycling stability. One of the most effective strategies is to polymerize small active molecules into polymers that are insoluble in electrolytes. Among various polymeric systems, polyimides (PIs), a class of organic carbonyl polymers, seem to be one of the promising electrode materials owing to their satisfying capacity, excellent cycling performance, and good rate capability. Moreover, they are structurally adjustable, safe when fully charged, and environmentally friendly.
In addition, another lithium-ion storage mechanism is like graphite by introducing large amounts of aromatic structures. This mechanism has been found by many references [104,105,106], and they utilize the conjugated benzenes to store lithium ions.
Similarly, PI active storage materials can also be divided into the cathode and anode, two types. As the cathode active storage materials, the lithium-ion storage mechanism is depending on the reversible reduction reactions of carbonyls in PI repeat units. This reaction mainly takes place at 1.5 ~ 3.5 V. Presently, the most widely used dianhydrides are pyromellitic dianhydrides (PMDA), naphthalene-tetracarboxylic dianhydrides (NTCDA), and perylene-tetracarboxylic dianhydrides (PTCDA). Much effort has been devoted to exploring the relationship between PI structure and electrochemical performances. Hernández et al. [107] synthesized many different PI active storage materials by the polycondensations of PMDA, NTCDA, and 4,4’-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) with various diamines shown in Fig. 10a. They utilized these PI active storage materials to assemble LIBs, whose structure is schematically shown in Fig. 10b. As NTCDA-PIs possess good conjugation structure, it shows better cycling performance and rate capability than PMDA-PIs and 6FDA-PIs. Then, NTCDA was used as the main dianhydride to synthesize a series of PI active storage materials, whose rate capability performances are shown in Fig. 10c. Firstly, NTCDA was polymerized with different aromatic diamines such as 3,3-bis-(4-aminophenyl)phthalide (APh), 3,3-bis-(4-aminophenyl)phthalimidine (APhl), 9,9-Bis-(4-aminophenyl)anthrone (AAn), and 3,3-bis(4-aminophenyl)quinuclidine) (AQn-LFSI), where NTCDA-APh PIs exhibit the best rate capability (146 mAh g−1 at 25 mA g−1, 100% of its theoretical capacity), as it took place the three-electron reaction where two electrons were from the imide part and the additional one from the extra carbonyl group of diamine. NTCDA-AAn PIs only take place the two-electron reactions (96 mAh g−1 at 25 mA g−1, 73% of its theoretical capacity) as the extra carbonyl group was not redox-active. Similarly, NTCDA-APhl PIs also take place the two-electron reactions (107 mAh g−1 at 25 mA g−1, 73% of its theoretical capacity) as the extra amide moiety was not redox-active. Additionally, when the ionic group is introduced in the structure of PIs as NTDA-AQn-TFSI, it can deliver the specific capacity of 90 mAh g−1, suggesting that the ionic moiety is not able to improve the rate capability of the polymer. They also investigated PIs synthesized with different aliphatic diamines including 4,7,10-trioxa-1,13-tridecanediamine (TOTDA), 4,9-dioxa-1,12-dodecanediamine (DODDA), and 1,12-dodecanediamine (DDA) and found that the NTCDA-DODDA PIs and NTCDA-TOTDA PIs exhibit better rate capability than NTCDA-DDA PIs due to the presence of oxyethylene fragments which is known to solvate lithium ions and improve the ionic conductivity. Therefore, the conjugation structure, redox-active carbonyl group, and oxyethylene fragments are three kinds of important structural units to tune the electrochemical performance.
Moreover, using redox-active anthraquinone units is also an effective strategy to design conjugated carbonyl polymers. Ba et al. [108] prepared the PMDA and NTCDA styles of PIs with another new diamine monomer containing a benzoquinone unit (AQPDA) shown in Fig. 11a. The specific capacity of these two PI cathodes at 0.1 C is 170 and 145 mAh g−1, respectively. The reason that PIs can be used as active materials is that active carbonyl groups introduced in molecular structures are more easily combined with lithium ions, playing the role of delivering lithium ions. Wang et al. [109] used a novel conjugated diketone to react with NTCDA to obtain a kind of multi-carbonyl PI cathode active storage material that shows a very stable cycling performance as shown in Fig. 11b. This PI cathode can deliver the specific capacity of 213 mAh g−1 (utilization of active sites is 78.7%) and the maximum energy density of 490 Wh kg−1, higher than traditional LIBs (300 Wh kg−1), demonstrating the significant application value.
Additionally, the stacking structure of polyimides is important in determining electrochemical performance. Gannett et al. [110] used the strongest conjugation structure of PTCDA to react with para-phenylenediamine (pPDA), trans-1,4-diaminocyclohexane (chex), and 1,2-ethylenediamine (en) to obtain polyimides with different crystallinity, flexibility, and stacking mode shown in Fig. 12a. The results demonstrate that PTCDA-en possesses rapid Li-ion conductivity due to alkyl linking units endow that with an amorphous structure. PTCDA-en possesses the smallest activation energy of charge transfer and diffusion-limited current shown in Fig. 12b, demonstrating that suitable packing structure should be also considered.
a Two one-electron reductions of diimides and the synthesis process of polyimides by tetracarboxylic acid dianhydrides and amine. b The charge compensation mechanisms for PTCDA-pPDA, PTCDA-chex, and PTCDA-en as cathodes in LIBs as well as CV tests of activation energy of charge transfer with Li-ion and diffusion-limited current proportion in Li+ and K+ batteries for the PDI-based polymers
When PI active storage materials are used as anodes, the lithium-ion storage mechanism is mainly attributed to the “hyperlithiation” phenomenon. The suitable operating voltage is at 0 ~ 1 V. More benzenes introduced to the PI repeat unit are beneficial to lithium-ion storage. As shown in Fig. 13a (i), He et al. [111] used PMDA and terephthalate (TA) to synthesize the PI active storage materials with six carbonyl groups per repeat unit. The as-prepared PI materials can be lithiated with 22 lithium ions, displaying the theoretical specific capacity of 1704 mAhg−1. CV test results shown in Fig. 13a (ii) confirmed the existence of enolization reactions of carbonyls and the “hyperlithiation” phenomenon. In the cycling process shown in Fig. 13a (iii), the specific capacity will increase, indicating the “hyperlithiation” process has taken place and the specific capacity reaches the maximum value. After this extremum, the specific capacity began to decrease. Changing the repeat units of PIs, the reversible carbonyl reduction reactions and the “hyperlithiation” process will occur, whose process is also trapped by the work of Liao et al. [112] in Fig. 13b(i-iii). Moreover, the ordered and conjugated structure of PIs, such as covalent organic framework, can form many lithium-ion storage channels and accommodate 17 lithium ions per repeating unit during the lithiation process, further enhancing the cycling performance [113] shown in Fig. 13c (i, ii).
To further improve the cycling and rate capacity, different kinds of conducting materials, such as graphene, carbon nanotubes, and carbon black, were compounded with PI active storage materials to prepare electrodes. Much effort is utilizing graphene to synthesize the PI-Graphene electrode materials by the in situ polymerization strategy, which could make full use of the surface area of graphene and possess several magnitudes higher electronic conductivity compared to pure PI polymers. In Fig. 14a, the graphene and two kinds of carbonyl polymers poly(anthraquinonyl) sulfide (PAQS) and polyimide (PI) were blended to prepare graphene/PI nanocomposites by the in situ polymerization method [98]. In Fig. 14b, the monolithic 3D graphene/polyimide composites (GF-PI) were prepared by the one-step solvothermal strategy [114]. In Fig. 14c, Ahmad et al. [115] developed a novel PI-FLEG (few-layer exfoliated graphene) nanocomposite, where PI nano-flakes shorten the diffusion path length between the electrolyte and electrode interfaces, leading to a further increase in the rate capability. In Fig. 14d, NTCDA and 2,6-diamino-anthraquinone (DAQ) as redox-active monomers in the presence of graphene oxide (GO) were in situ polymerized to obtain the graphene-sandwiched PQI nanosheets (PQI@Gr) [116]. Obviously, graphene is a promising conductive material.
Apart from graphene, carbon nanotubes and carbon black are also reported to be used in the preparation of active storage materials. Combining a sequential assembly and high-temperature dehydration strategies, carbon nanotube (CNT)/polyimide (PI)/mesoporous Fe2O3 (meso-Fe2O3/PI/CNT) ternary nanocomposite materials were prepared by Ban et al. [117] as shown in Fig. 15a. Zhang et al. [118] fabricated the composites of polyimide and carbon black (PI/CBs) by the in situ condensations of PMDA and pPDA with the presence of carbon black shown in Fig. 15b. Due to the high conductive property of carbon materials, the capacity of PI active storage materials can be effectively utilized, further increasing the energy density. Currently, increasing the specific capacity and operating voltage are two effective strategies to increase energy density. Nevertheless, the average operating voltage is limited by the intrinsic structures of PI molecules, which are less than 3.5 V usually. Designing structures that can withstand high voltage is challenging. Therefore, increasing the specific capacity is the first choice. The effective method is by introducing more carbonyls or conjugated structures which are beneficial to increase the lithium-ion storage capacity and further increase the energy density.
3 Perspectives on Using PIs in Practical LIBs
PIs have many advantages, including excellent mechanical properties, outstanding thermal stability, satisfactory electrolyte wettability, and good (electro)chemical resistance, which are promising candidates for coatings, separators, binders, solid-state electrolytes, and active storage materials. Appropriate PIs need to be selected based on application requirements. This section summarizes the main issues that PIs can address in current LIBs and proposes strategies for researchers in developing PIs for LIBs. Figure 16 lists the strategies for PIs applied in LIBs.
3.1 PI Coatings
The coating is an effective way to improve the performance of high-voltage cathodes. To date, only a few studies have reported PI coatings, even though PIs have some advantages over traditional inorganic materials. The outstanding features of PIs such as designable molecular structures, continuous texture, and high-voltage/high-temperature resistance all attract research interest.
PI coatings can be implemented in three ways. First, researchers could use monomers with different structures to synthesize PI coatings with targeted performance. Second, to improve the electrochemical performance, inorganic materials (such as Al2O3, ZrO2, and TiO2), electron-conducting materials (such as graphene, carbon nanotubes, and carbon black), and their mixtures can be compounded with PIs to achieve co-coating layers [119, 120]. Third, self-healing, heat conductive, fluorescence, etc., functions can be introduced to PIs to realize the functionalization of cathode materials. Figure 17 shows the design strategies of PI coatings.
Moreover, PI coatings are not only limited to cathodes, but they can also coat silicon anodes [121]. These all deserve to explore further.
3.2 PI Separators
The separators play a vital role in the safety of LIBs. Once the separator is destroyed due to thermal shrinkage, ISCs may happen, causing thermal runaway. Therefore, LIBs have strict requirements for separator performance. First, the separator should possess high mechanical strength to separate the cathode and anode under high tensile and compressive stress. Second, the separator should be thermally stable with little or limited thermal shrinkage (< 5%). Further, the separator needs to be wettable by the electrolyte. Moreover, its pore distribution should be uniform to allow lithium ions to pass through. Finally, the separator should be highly insulating. Although polyethylene separators have been commercialized, their high thermal shrinkage and low trigger temperatures cause poor safety in LIBs. Currently, there are three ways to enhance the thermal stability of polyolefin separators: (1) using PP/PE/PP composite separators; (2) coating polyolefin separators with thermally stable inorganics; and (3) coating polyolefin separators with thermally stable polymers. However, these approaches still cannot satisfy the requirements for safe batteries.
The PI separators can improve safety in LIBs. However, PIs have only been used in research laboratories due to three reasons: They absorb water and are expensive and difficult to process. Choosing hydrophobic monomers or designing hydrophobic structures can address the water absorption issue. To decrease costs, researchers should devote effort to reducing raw material costs and improving processing equipment. The most critical issue in processing is to achieve uniform pore distribution. Two strategies to solve the issue of pore-making can be followed. The first is by blending PI and polyolefin materials to obtain a composite separator. This approach utilizes the good processability of polyolefins to achieve uniform and well-distributed pores during heating and stretching. PIs contribute to electrolyte wettability, mechanical strength, and thermal stability. Second, a pure semi-crystalline PI can be designed and prepared. The separator can be obtained by stretching and orienting at its melting point. These approaches all require improvements in the preparation capability of the processing equipment, especially at high temperatures. Figure 18 shows the processing techniques for PI separators.
3.3 PI Binders
Large volume expansion and shrinkage during cycling in silicon anodes cause rapid decay of electrochemical performance. Elastic binders can constrain silicon particles to avoid pulverization and electrical contact loss. However, PI binders with 3D crosslinking networks can maintain the anode integrity while accommodating high silicon active material content. The adhesive properties of the anode also need to be enhanced to prevent silicon anode pulverization. Enhancement could be achieved by introducing adhesive functional groups such as hydrogen bonds or metal–ligand coordination interactions in the binder. Besides these conventional approaches, multi-functionalization can also be one of the future directions for PI binders. For example, electronic or Li-ion conducting functional groups can be introduced to compensate for the limited electronic or ionic conduction. Safety and lifespan should also be considered. In summary, functional binders are a crucial research topic.
In fact, there are some references reported on PI binders for cathodes [79, 80, 122,123,124]. Several important references [80, 122,123,124] are from the group of Qi et al. to explore high-voltage performance. The above-modified strategies are also fit for PI cathode binders. Figure 19 shows the design strategies of PI binders.
3.4 PI Solid-State Electrolytes
The PI-based SSEs may be essential in all-solid-state LIBs because they can function as a separator and an electrolyte. Apart from this, there are three methods for designing PI SSEs. First, traditional separators can be coated with PI solutes and lithium salts or ceramic particles. Second, PI itself can be used as the separator matrix with other polymer solutes, lithium salts, and ceramic particles coated on its surface. Third, PI separator is coated with PI solutes and lithium salts or ceramic particles. Figure 20 shows the design strategies of PI SSEs.
3.5 PIs Active Storage Materials
In recent years, a variety of organic active storage materials have developed rapidly, among which PIs with stable structures, large theoretical capacity, good heat resistance, high tensile modulus, and high safety have attracted wide attention. However, there are still some problems in the practical application of PI active storage materials including the low utilization rate of redox-active carbonyl groups and poor electronic conductivity. To solve these problems, the following two strategies are adopted. Firstly, the reasonable structure design of PI active storage materials should be promoted. The energy storage mechanism of active storage materials is closely related to the conjugated structures and redox-active sites. Utilizing the large π–π packing structures is encouraged, and more redox-active functional groups should be introduced such as the disulfide bond (S–S), azo bond (N = N), and imine bond (C = N) besides carbonyl groups (C = O). Furthermore, ordered structures such as popular covalent organic framework compounds (COFs) linked by imine units should be considered. Secondly, more advancing electron-conducting materials should be investigated and introduced to the PI active storage materials to enhance the energy utilization rate of PIs. Figure 21 shows the design strategies of PI active storage materials.
4 Outlook and Perspective
In this perspective, we presented the recent progress in PI coatings, separators, binders, SSEs, and active storage materials, revealing the potential of PIs in improving LIB safety and electrochemical performance. We identified several strategies to further improve LIBs using PIs. In summary, future works on implementing PIs in LIBs should consider the following three points:
-
(1)
Devoting much effort to designing the PI molecular structure and choosing suitable preparation methods for critical PI components according to LIB requirements.
-
(2)
Advancing functional PI and PI composites to decrease cost and improve LIB energy density, rate capacity, cycling stability, and safety.
-
(3)
Developing practical processing technologies and accelerating industrialization to implement PI components in LIBs.
Promoting the development of new PI materials and taking advantage of performance benefits from these new materials advances LIBs. Next-generation LIBs can be economical, environmentally benign, and safe to use. Meanwhile, large-scale LIBs utilization can relieve the energy crisis. Hence, high-performance LIBs could advance our connected world toward a more sustainable future.
References
C. Zou, Q. Zhao, G. Zhang, B. Xiong, Energy revolution: From a fossil energy era to a new energy era. Nat. Gas Ind. B 3(1), 1–11 (2016). https://doi.org/10.1016/j.ngib.2016.02.001
Y. Sun, Q. Wu, G. Shi, Graphene based new energy materials. Energy Environ. Sci. 4(4), 1113–1132 (2011). https://doi.org/10.1039/C0EE00683A
A. Manthiram, An outlook on lithium ion battery technology. ACS Central Sci. 3(10), 1063–1069 (2017). https://doi.org/10.1021/acscentsci.7b00288
G. Zubi, R. Dufo-López, M. Carvalho, G. Pasaoglu, The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 89, 292–308 (2018). https://doi.org/10.1016/j.rser.2018.03.002
B. Scrosati, Recent advances in lithium ion battery materials. Electrochim. Acta 45(15–16), 2461–2466 (2000). https://doi.org/10.1016/S0013-4686(00)00333-9
L. Lu, X. Han, J. Li, J. Hua, M. Ouyang, A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 226, 272–288 (2013). https://doi.org/10.1016/j.jpowsour.2012.10.060
A. Jansen, A. Kahaian, K. Kepler, P. Nelson, K. Amine et al., Development of a high-power lithium-ion battery. J. Power Sources 81, 902–905 (1999). https://doi.org/10.1016/S0378-7753(99)00268-2
J. Chen, Recent progress in advanced materials for lithium ion batteries. Materials 6(1), 156–183 (2013). https://doi.org/10.3390/ma6010156
G. Blomgren, The development and future of lithium ion batteries. J. Electrochem. Soc. 164(1), A5019–A5025 (2016). https://doi.org/10.1149/2.0251701jes
C. Costa, E. Lizundia, S. Lanceros-Méndez, Polymers for advanced lithium-ion batteries: state of the art and future needs on polymers for the different battery components. Prog. Energy Combust. Sci. 79, 100846–100880 (2020). https://doi.org/10.1016/j.pecs.2020.100846
J. Li, Y. Cai, H. Wu, Z. Yu, X. Yan et al., Polymers in lithium-ion and lithium metal batteries. Adv. Energy Mater. 11(15), 2003239 (2021). https://doi.org/10.1002/aenm.202003239
A. Sezer Hicyilmaz, A. Celik Bedeloglu, Applications of polyimide coatings: A review. SN Appl. Sci. 3(3), 363–385 (2021). https://doi.org/10.1007/s42452-021-04362-5
C. Ye, M. Liu, X. Zhang, Q. Tong, M. Zhu et al., Review-long-term cyclability of high-temperature stable polyimide in libs. J. Electrochem. Soc. 168(10), 100519–100538 (2021). https://doi.org/10.1149/1945-7111/ac28c4
H. Yu, Y. Shi, B. Yuan, Y. He, L. Qiao et al., Recent developments of polyimide materials for lithium-ion battery separators. Ionics 27(3), 907–923 (2021). https://doi.org/10.1007/s11581-020-03865-2
N. DeLuca, Y. Elabd, Polymer electrolyte membranes for the direct methanol fuel cell. A Rev. J. Polym. Sci. Part B Polym. Phys. 44(16), 2201 (2006). https://doi.org/10.1002/polb.20861
Z. Song, H. Zhan, Y. Zhou, Polyimides: Promising energy-storage materials. Angew. Chem. Int. Ed. 49(45), 8444–8448 (2010). https://doi.org/10.1002/anie.201002439
M. Ding, Polyimide materials: Chemistry, Relationship between Structure and Properties and Materials, 2nd edn. (Science Press, Beijing, 2006), pp.9–53
D.J. Liaw, K.L. Wang, Y.C. Huang, K.R. Lee, J.Y. Lai et al., Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 37(7), 907–974 (2012). https://doi.org/10.1016/j.progpolymsci.2012.02.005
C. Liu, F. Li, L.P. Ma, H.M. Cheng, Crystalline materials: colloidal-crystal-assisted patterning of crystalline materials advanced materials for energy storage. Adv. Mater. 22(8), E28–E62 (2010). https://doi.org/10.1002/adma.200903328
J.W. Fergus, Recent developments in cathode materials for lithium ion batteries. J. Power Sources 195(4), 939–954 (2010). https://doi.org/10.1016/j.jpowsour.2009.08.089
J. Xu, S. Dou, H. Liu, L. Dai, Cathode materials for next generation lithium ion batteries. Nano Energy 2(4), 439–442 (2013). https://doi.org/10.1016/j.nanoen.2013.05.013
F. Schipper, P.K. Nayak, E.M. Erickson, S.F. Amalraj, O. Srur-Lavi et al., Study of cathode materials for lithium-ion batteries: recent progress and new challenges. Inorganics 5(2), 32 (2017). https://doi.org/10.3390/inorganics5020032
F. Lin, I.M. Markus, D. Nordlund, T.C. Weng, M.D. Asta et al., Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5(1), 3529 (2014). https://doi.org/10.1038/ncomms4529
S. Liu, Z. Wang, Y. Huang, Z. Ni, J. Bai et al., Fluorine doping and Al2O3 coating Co-modified Li[Li0.20Ni0.133Co0.133Mn0.534]O2 as high performance cathode material for lithium-ion batteries. J. Alloys Compd. 731, 636–645 (2018)
N. Su, Y. Lyu, R. Gu, B. Guo, Al2O3 coated Li1.2Ni0.2Mn0.2Ru0.4O2 as cathode material for Li-ion batteries. J. Alloys Compd. 741, 398–403 (2018). https://doi.org/10.1016/j.jallcom.2018.01.146
N. Dannehl, S.O. Steinmüller, D.V. Szabó, M. Pein, F. Sigel et al., High-resolution surface analysis on aluminum oxide-coated Li1.2Mn0.55Ni0.15Co0.1O2 with improved capacity retention. ACS Appl. Mater. Interf. 10(49), 43131–43143 (2018). https://doi.org/10.1021/acsami.8b09550
G. Kobayashi, Y. Irii, F. Matsumoto, A. Ito, Y. Ohsawa et al., Improving cycling performance of Li-rich layered cathode materials through combination of Al2O3-based surface modification and stepwise precycling. J. Power Sources 303, 250–256 (2016). https://doi.org/10.1016/j.jpowsour.2015.11.014
Z. Wang, E. Liu, L. Guo, C. Shi, C. He et al., Cycle performance improvement of Li-rich layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by ZrO2 coating. Surf. Coat. Technol. 235, 570–576 (2013). https://doi.org/10.1016/j.surfcoat.2013.08.026
F. Wu, Z. Wang, Y. Su, N. Yan, L. Bao et al., Li[Li0.2Mn0.54Ni0.13Co0.13]O2-MoO3 composite cathodes with low irreversible capacity loss for lithium ion batteries. J. Power Sources 247, 20–25 (2014). https://doi.org/10.1016/j.jpowsour.2013.08.031
J.M. Zheng, J. Li, Z.R. Zhang, X.J. Guo, Y. Yang, The effects of TiO2 coating on the electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium-ion battery. Solid State Ionics 179(27), 1794–1799 (2008). https://doi.org/10.1016/j.ssi.2008.01.091
Y.K. Sun, M.J. Lee, C.S. Yoon, J. Hassoun, K. Amine et al., The role of AlF3 coatings in improving electrochemical cycling of Li-enriched nickel-manganese oxide electrodes for Li-ion batteries. Adv. Mater. 24(9), 1192–1196 (2012). https://doi.org/10.1002/adma.201104106
H.G. Song, J.Y. Kim, K.T. Kim, Y.J. Park, Enhanced electrochemical properties of Li(Ni0.4Co0.3Mn0.3)O2 cathode by surface modification using Li3PO4-based materials. J. Power Sources 196(16), 6847–6855 (2011). https://doi.org/10.1016/j.jpowsour.2010.09.027
S.Y. Lee, J.H. Park, J.H. Cho, S.B. Kim, W.S. Kim et al., A novel ion-conductive protection skin based on polyimide gel polymer electrolyte: application to nanoscale coating layer of high voltage LiNi1/3C1/3Mn1/3O2 cathode materials for lithium-ion batteries. J. Mater. Chem. 22(25), 12574–12581 (2012). https://doi.org/10.1039/c2jm16799a
J.H. Park, J.H. Cho, J.S. Kim, E.G. Shim, S.Y. Lee, High-voltage cell performance and thermal stability of nanoarchitectured polyimide gel polymer electrolyte-coated LiCoO2 cathode materials. Electrochim. Acta 86, 346–351 (2012). https://doi.org/10.1016/j.electacta.2012.04.073
J. Zhang, Q. Lu, J. Fang, J. Wang, J. Yang et al., Polyimide encapsulated lithium-rich cathode material for high voltage lithium-ion battery. ACS Appl. Mater. Interfaces 6(20), 17965–17973 (2014). https://doi.org/10.1021/am504796n
Y. Wang, Separator wettability enhanced by electrolyte additive to boost the electrochemical performance of lithium metal batteries. Nano-Micro Lett. 13, 210 (2021). https://doi.org/10.1007/s40820-021-00731-2
X. Huang, Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 15(4), 649–662 (2011). https://doi.org/10.1007/s10008-010-1264-9
M.F. Lagadec, R. Zahn, V. Wood, Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 4(1), 16–25 (2019). https://doi.org/10.1038/s41560-018-0295-9
Z. Liu, Y. Jiang, Q. Hu, S. Guo, L. Yu et al., Materials Safer lithium-ion batteries from the separator aspect Development and future perspectives. Energy Environ. Mater 4(3), 336–362 (2021). https://doi.org/10.1002/eem2.12129
G. Dong, N. Dong, B. Liu, G. Tian, S. Qi et al., Ultrathin inorganic-nanoshell encapsulation: TiO2 coated polyimide nanofiber membrane enabled by layer-by-layer deposition for advanced and safe high-power LIB separator. J. Membr. Sci. 601, 117884–117892 (2020). https://doi.org/10.1016/j.memsci.2020.117884
W. Jiang, Z. Liu, Q. Kong, J. Yao, C. Zhang et al., A high temperature operating nanofibrous polyimide separator in li-ion battery. Solid State Ionics 232, 44–48 (2013). https://doi.org/10.1016/j.ssi.2012.11.010
L. He, J.H. Cao, T. Liang, D.Y. Wu, Effect of monomer structure on properties of polyimide as lib separator and its mechanism study. Electrochim. Acta 337, 135838–135848 (2020). https://doi.org/10.1016/j.electacta.2020.135838
D. Lin, D. Zhuo, Y. Liu, Y. Cui, All-integrated bifunctional separator for li dendrite detection via novel solution synthesis of a thermostable polyimide separator. J. Am. Chem. Soc. 138(34), 11044–11050 (2016). https://doi.org/10.1021/jacs.6b06324
M. Li, Z. Zhang, Y. Yin, W. Guo, Y. Bai et al., Novel polyimide separator prepared with two porogens for safe lithium-ion batteries. ACS Appl. Mater. Interfaces 12(3), 3610–3616 (2020). https://doi.org/10.1021/acsami.9b19049
P. Zhou, D. Yao, H. Liang, J. Yin, Y. Xia et al., Highly connective spongy polyimide separators blended with inorganic whiskers for high-performance lithium-ion batteries. ACS Appl. Energy Mater. 5(2), 2011–2023 (2022). https://doi.org/10.1021/acsaem.1c03548
Y. Song, X. Liu, D. Ren, H. Liang, L. Wang et al., Simultaneously blocking chemical crosstalk and internal short circuit via gel-stretching derived nanoporous non-shrinkage separator for safe lithium-ion batteries. Adv. Mater. 34(2), 2106335–2106345 (2021). https://doi.org/10.1002/adma.202106335
J. Li, K. Luo, J. Yu, Y. Wang, J. Zhu et al., Promising free-standing polyimide membrane via solution blow spinning for high performance lithium-ion batteries. Ind. Eng. Chem. Res. 57(36), 12296–12305 (2018). https://doi.org/10.1021/acs.iecr.8b02755
D. Wu, N. Dong, R. Wang, S. Qi, B. Liu et al., In situ construction of high-safety and non-flammable polyimide “ceramic” lithium-ion battery separator via SiO2 nano-encapsulation. Chem. Eng. J. 420, 129992–130001 (2021). https://doi.org/10.1016/j.cej.2021.129992
N. Dong, J. Wang, N. Chen, B. Liu, G. Tian et al., In situ reinforcing: ZrO2-armored hybrid polyimide separators for advanced and safe lithium-ion batteries. ACS Sustain. Chem. Eng. 9(18), 6250–6257 (2021). https://doi.org/10.1021/acssuschemeng.0c08818
M. Qiao, G. Zhang, J. Deng, J. Guo, J.J. Zhang, Electrospun polyimide@ organic-montmorillonite composite separator with enhanced mechanical and thermal performances for high-safety lithium-ion battery. J. Mater. Sci. 57(25), 11796–11808 (2022). https://doi.org/10.1007/s10853-022-07343-0
M. Holzapfel, H. Buqa, L.J. Hardwick, M. Hahn, A. Würsig et al., Nano silicon for lithium-ion batteries. Electrochim. Acta 52(3), 973–978 (2006). https://doi.org/10.1016/j.electacta.2006.06.034
H. Li, A high capacity nano-Si composite anode material for lithium rechargeable batteries. Electrochem. Solid-State Lett. 2(11), 547–549 (1999). https://doi.org/10.1149/1.1390899
S. You, H. Tan, L. Wei, W. Tan, C. Li, Design strategies of Si/C composite anode for lithium-ion batteries. Chem. Eur. J. 27(48), 12237–12256 (2021). https://doi.org/10.1002/chem.202100842
R. Yi, J. Zai, F. Dai, M.L. Gordin, D. Wang, Dual conductive network-enabled graphene/Si-C composite anode with high areal capacity for lithium-ion batteries. Nano Energy 6, 211–218 (2014). https://doi.org/10.1016/j.nanoen.2014.04.006
X. Gao, W. Lu, J. Xu, Insights into the li diffusion mechanism in Si/C composite anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 13(18), 21362–21370 (2021). https://doi.org/10.1021/acsami.1c03366
Y. Shi, X. Zhou, G. Yu, Material and structural design of novel binder systems for high-energy, high-power lithium-ion batteries. Acc. Chem. Res. 50(11), 2642–2652 (2017). https://doi.org/10.1021/acs.accounts.7b00402
J. Li, R.B. Lewis, J.R. Dahn, Sodium carboxymethyl cellulose: a potential binder for Si negative electrodes for Li-ion batteries. Electrochem. Solid-State Lett. 10(2), A17–A20 (2007). https://doi.org/10.1149/1.2398725
A. Magasinski, B. Zdyrko, I. Kovalenko, B. Hertzberg, R. Burtovyy et al., Toward efficient binders for Li-ion battery Si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2(11), 3004–3010 (2010). https://doi.org/10.1021/am100871y
J.S. Bridel, T. Azaïs, M. Morcrette, J.M. Tarascon, D. Larcher, Key parameters governing the reversibility of si/carbon/cmc electrodes for li-ion batteries. Chem. Mater. 22(3), 1229–1241 (2010). https://doi.org/10.1021/cm902688w
D. Shao, H. Zhong, L. Zhang, Water-soluble conductive composite binder containing PEDOT:PSS as conduction promoting agent for si anode of lithium-ion batteries. Chem Electro Chem 1(10), 1679–1687 (2014). https://doi.org/10.1002/celc.201402210
C. Wang, H. Wu, Z. Chen, M.T. McDowell, Y. Cui et al., Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 5(12), 1042–1048 (2013). https://doi.org/10.1038/nchem.1802
M.H. Ryou, J. Kim, I. Lee, S. Kim, Y.K. Jeong et al., Mussel-inspired adhesive binders for high-performance silicon nanoparticle anodes in lithium-ion batteries. Adv. Mater. 25(11), 1571–1576 (2013). https://doi.org/10.1002/adma.201203981
H. Zhao, Y. Wei, C. Wang, R. Qiao, W. Yang et al., Mussel-inspired conductive polymer binder for si-alloy anode in lithium-ion batteries. ACS Appl. Mater. Interfaces 10(6), 5440–5446 (2018). https://doi.org/10.1021/acsami.7b14645
R. Wang, D. Feng, T. Chen, S. Chen, Y. Liu, Mussel-inspired polydopamine treated Si/C electrode as high-performance anode for lithium-ion batteries. J. Alloys Compd. 825, 154081–154090 (2020). https://doi.org/10.1016/j.jallcom.2020.154081
J.H. Lee, U. Paik, V.A. Hackley, Y.M. Choi, Effect of poly(acrylic acid) on adhesion strength and electrochemical performance of natural graphite negative electrode for lithium-ion batteries. J. Power Sources 161(1), 612–616 (2006). https://doi.org/10.1016/j.jpowsour.2006.03.087
M. Murase, N. Yabuuchi, Z.-J. Han, J.-Y. Son, Y.-T. Cui et al., Crop-derived polysaccharides as binders for high-capacity silicon/graphite-based electrodes in lithium-ion batteries. ChemSusChem 5(12), 2307–2311 (2012). https://doi.org/10.1002/cssc.201200650
S. Hu, Z. Cai, T. Huang, H. Zhang, A. Yu, A modified natural polysaccharide as a high-performance binder for silicon anodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 11(4), 4311–4317 (2019). https://doi.org/10.1021/acsami.8b15695
L. Gong, M.H.T. Nguyen, E.-S. Oh, High polar polyacrylonitrile as a potential binder for negative electrodes in lithium ion batteries. Electrochem. Commun. 29, 45–47 (2013). https://doi.org/10.1016/j.elecom.2013.01.010
N. Ohta, T. Sogabe, K. Kuroda, A novel binder for the graphite anode of rechargeable lithium ion batteries for the improvement of reversible capacity. Carbon 39(9), 1434–1436 (2001). https://doi.org/10.1016/S0008-6223(01)00079-3
C.H. Tsao, T.K. Yang, K.Y. Chen, C.E. Fang, M. Ueda et al., Comparing the ion-conducting polymers with sulfonate and ether moieties as cathode binders for high-power lithium-ion batteries. ACS Appl. Mater. Interfaces 13(8), 9846–9855 (2021). https://doi.org/10.1021/acsami.0c20657
J.S. Kim, W. Choi, K.Y. Cho, D. Byun, J. Lim et al., Effect of polyimide binder on electrochemical characteristics of surface-modified silicon anode for lithium ion batteries. J. Power Sources 244, 521–526 (2013). https://doi.org/10.1016/j.jpowsour.2013.02.049
Z. Xu, J. Yang, T. Zhang, Y. Nuli, J. Wang et al., Silicon microparticle anodes with self-healing multiple network binder. Joule 2(5), 950–961 (2018). https://doi.org/10.1016/j.joule.2018.02.012
T.M. Higgins, S.H. Park, P.J. King, C. Zhang, N. McEvoy et al., A commercial conducting polymer as both binder and conductive additive for silicon nanoparticle-based lithium-ion battery negative electrodes. ACS Nano 10(3), 3702–3713 (2016). https://doi.org/10.1021/acsnano.6b00218
H. Zhao, Z. Wang, P. Lu, M. Jiang, F. Shi et al., Toward practical application of functional conductive polymer binder for a high-energy lithium-ion battery design. Nano Lett. 14(11), 6704–6710 (2014). https://doi.org/10.1021/nl503490h
D. Yao, Y. Yang, Y. Deng, C. Wang, Flexible polyimides through one-pot synthesis as water-soluble binders for silicon anodes in lithium ion batteries. J. Power Sources 379, 26–32 (2018). https://doi.org/10.1016/j.jpowsour.2017.12.086
L. Ma, J.Q. Meng, Y.J. Cheng, Q. Ji, X. Zuo et al., Poly(siloxane imide) binder for silicon-based lithium-ion battery anodes via rigidness/softness coupling. Chem. Asian J. 15(17), 2674–2680 (2020). https://doi.org/10.1002/asia.202000633
J. Choi, K. Kim, J. Jeong, K.Y. Cho, M.H. Ryou et al., Highly adhesive and soluble copolyimide binder: Improving the long-term cycle life of silicon anodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 7(27), 14851–14858 (2015). https://doi.org/10.1021/acsami.5b03364
Y. Xu, Q. Zhang, N. Lv, H. Li, Z. Wei et al., Carboxyl function group introduced to polyimide binders for silicon anode materials. Energy Fuels 37(3), 2441–2448 (2023). https://doi.org/10.1021/acs.energyfuels.2c04024
H.Q. Pham, J. Lee, H.M. Jung, S. Song, Non-flammable LiNi0.8CO0.1Mn0.1O2 cathode via functional binder; stabilizing high-voltage interface and performance for safer and high-energy lithium rechargeable batteries. Electrochim. Acta 317, 711–721 (2019). https://doi.org/10.1016/j.electacta.2019.06.034
K. Qi, Y. Wang, N. Dong, B. Liu, G. Tian et al., Novel polyimide binders integrated with soft and hard functional segments ensuring long-term high-voltage operating stability of high-energy NCM811 lithium-ion batteries up to 4.5 V. Appl. Energy 320, 119282 (2022). https://doi.org/10.1016/j.apenergy.2022.119282
Q. Zhao, S. Stalin, C.Z. Zhao, L.A. Archer, Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 5(3), 229–252 (2020). https://doi.org/10.1038/s41578-019-0165-5
T. Famprikis, P. Canepa, J.A. Dawson, M.S. Islam, C. Masquelier, Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 18(12), 1278–1291 (2019). https://doi.org/10.1038/s41563-019-0431-3
A. Manthiram, X. Yu, S. Wang, Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2(4), 16103 (2017). https://doi.org/10.1038/natrevmats.2016.103
J. Lee, C.L. Lee, K. Park, I.D. Kim, Synthesis of an Al2O3-coated polyimide nanofiber mat and its electrochemical characteristics as a separator for lithium ion batteries. J. Power Sources 248, 1211–1217 (2014). https://doi.org/10.1016/j.jpowsour.2013.10.056
M. Higa, K. Yaguchi, R. Kitani, All solid-state polymer electrolytes prepared from a graft copolymer consisting of a polyimide main chain and poly(ethylene oxide) based side chains. Electrochim. Acta. 55(4), 1380–1384 (2010). https://doi.org/10.1016/j.electacta.2009.07.046
J. Wan, J. Xie, X. Kong, Z. Liu, K. Liu et al., Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 14(7), 705–711 (2019). https://doi.org/10.1038/s41565-019-0465-3
Y. Cui, J. Wan, Y. Ye, K. Liu, L.Y. Chou et al., A fireproof, lightweight, polymer-polymer solid-state electrolyte for safe lithium batteries. Nano Lett. 20(3), 1686–1692 (2020). https://doi.org/10.1021/acs.nanolett.9b04815
J. Hu, P. He, B. Zhang, B. Wang, L.Z. Fan, Porous film host-derived 3D composite polymer electrolyte for high-voltage solid state lithium batteries. Energy Storage Mater. 26, 283–289 (2020). https://doi.org/10.1016/j.ensm.2020.01.006
J. Gai, F. Ma, Z. Zhang, D. Sun, Y. Jin et al., Flexible organic-inorganic composite solid electrolyte with asymmetric structure for room temperature solid-state Li-ion batteries. ACS Sustain. Chem. Eng. 7(19), 15896–15903 (2019). https://doi.org/10.1021/acssuschemeng.9b01869
X. Shen, T. Hu, Y. Zeng, X. Huang, P. Zhang et al., Core-shell structured gel polymer electrolyte with single-ion conducting and thermal stability bifunction for lithium-ion batteries. J. Electrochem. Soc. 169(7), 070505–070514 (2022). https://doi.org/10.1149/1945-7111/ac79d5
Y. Li, Z. Fu, S. Lu, X. Sun, X. Zhang et al., Polymer nanofibers framework composite solid electrolyte with lithium dendrite suppression for long life all-solid-state lithium metal battery. Chem. Eng. J. 440, 135816–135825 (2022). https://doi.org/10.1016/j.cej.2022.135816
Z. Song, Y. Qian, X. Liu, T. Zhang, Y. Zhu et al., A quinone-based oligomeric lithium salt for superior Li–organic batteries. Energy Environ. Sci. 7(12), 4077–4086 (2014). https://doi.org/10.1039/C4EE02575J
K. Liu, J. Zheng, G. Zhong, Y. Yang, Poly(2,5-dihydroxy-1,4-benzoquinonyl sulfide) (PDBS) as a cathode material for lithium ion batteries. J. Mater. Chem. 21(12), 4125–4131 (2011). https://doi.org/10.1039/C0JM03127E
T. Janoschka, M.D. Hager, U.S. Schubert, Powering up the future: Radical polymers for battery applications. Adv. Mater. 24(48), 6397–6409 (2012). https://doi.org/10.1002/adma.201203119
A.L. Goodwin, Packing down. Adv. Mater. 9(1), 7–8 (2010). https://doi.org/10.1038/nmat2597
B. Häupler, A. Wild, U.S. Schubert, Carbonyls: powerful organic materials for secondary batteries. Adv. Energy Mater. 5(11), 1402034 (2015). https://doi.org/10.1002/aenm.201402034
H.G. Wang, S. Yuan, D.L. Ma, X.L. Huang, F.L. Meng et al., Tailored aromatic carbonyl derivative polyimides for high-power and long-cycle sodium-organic batteries. Adv. Energy Mater. 4(7), 1301651–1301658 (2014). https://doi.org/10.1002/aenm.201301651
Z. Song, T. Xu, M.L. Gordin, Y.B. Jiang, I.T. Bae et al., Polymer-graphene nanocomposites as ultrafast-charge and -discharge cathodes for rechargeable lithium batteries. Nano Lett. 12(5), 2205–2211 (2012). https://doi.org/10.1021/nl2039666
C. Luo, X. Ji, S. Hou, N. Eidson, X. Fan et al., Azo compounds derived from electrochemical reduction of nitro compounds for high performance Li-ion batteries. Adv. Mater. 30(23), 1706498–1706507 (2018). https://doi.org/10.1002/adma.201706498
C. Luo, O. Borodin, X. Ji, S. Hou, K.J. Gaskell et al., Azo compounds as a family of organic electrode materials for alkali-ion batteries. Proc. Natl. Acad. Sci. USA 115(9), 2004–2009 (2018). https://doi.org/10.1073/pnas.1717892115
J. Wang, A.E. Lakraychi, X. Liu, L. Sieuw, C. Morari et al., Conjugated sulfonamides as a class of organic lithium-ion positive electrodes. Nat. Mater. 20(5), 665–673 (2021). https://doi.org/10.1038/s41563-020-00869-1
J. Wang, X. Guo, P. Apostol, X. Liu, K. Robeyns et al., High performance Li-, Na-, and K-ion storage in electrically conducting coordination polymers. Energy Environ. Sci. 15(9), 3923–3932 (2022). https://doi.org/10.1039/D2EE00566B
J. Wang, X. Liu, H. Jia, P. Apostol, X. Guo et al., A high-voltage organic framework for high-performance Na- and K-ion batteries. ACS Energy Lett. 7(2), 668–674 (2022). https://doi.org/10.1021/acsenergylett.1c02571
M. Ruby Raj, R.V. Mangalaraja, G. Lee, D. Contreras, K. Zaghib et al., Large π-conjugated condensed perylene-based aromatic polyimide as organic cathode for lithium-ion batteries. ACS Appl. Energy Mater. 3(7), 6511–6524 (2020). https://doi.org/10.1021/acsaem.0c00729
K.B. Labasan, H.-J. Lin, F. Baskoro, J.J.H. Togonon, H.Q. Wong et al., Dicyanotriphenylamine-based polyimides as high-performance electrodes for next generation organic lithium-ion batteries. ACS Appl. Mater. Interfaces 13(15), 17467–17477 (2021). https://doi.org/10.1021/acsami.1c00065
H. Yang, S. Liu, L. Cao, S. Jiang, H.Q. Hou, Superlithiation of non-conductive polyimide toward high-performance lithium-ion batteries. J. Mater. Chem. A 6(42), 21216–21224 (2018). https://doi.org/10.1039/C8TA05109G
G. Hernández, M. Salsamendi, S.M. Morozova, E.I. Lozinskaya, S. Devaraj et al., Polyimides as cathodic materials in lithium batteries: Effect of the chemical structure of the diamine monomer. J. Polym. Sci. A: Polym. Chem. 56(7), 714–723 (2018). https://doi.org/10.1002/pola.28937
Z. Ba, Z. Wang, M. Luo, H.-B. Li, Y. Li et al., Benzoquinone-based polyimide derivatives as high-capacity and stable organic cathodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 12(1), 807–817 (2019). https://doi.org/10.1021/acsami.9b18422
J. Wang, H. Liu, C. Du, X. Zhang, Y. Liu et al., Conjugated diketone-linked polyimide cathode material for organic lithium-ion batteries. Chem. Engin. J. 444, 136598 (2022). https://doi.org/10.1016/j.cej.2022.136598
C.N. Gannett, J. Kim, D. Tirtariyadi, P.J. Milner, H. Abruña, Investigation of ion-electrode interactions of linear polyimides and alkali metal ions for next generation alternative-ion batteries. Chem. Sci. 13(32), 9191–9201 (2022). https://doi.org/10.1039/D2SC02939A
J. He, Y. Liao, Q. Hu, Z. Zeng, L. Yi et al., Multi carbonyl polyimide as high capacity anode materials for lithium ion batteries. J. Power Sources 451, 227792 (2020). https://doi.org/10.1016/j.jpowsour.2020.227792
Y. Liao, J. He, L. Yi, Y. Tang, X. Li et al., Electrochemical kinetic study of a polyimide anode for lithium-ion batteries using the ac impedance technique. ACS Appl. Energy Mater. 4(5), 5348–5358 (2021). https://doi.org/10.1021/acsaem.1c00941
H. Kang, H. Liu, C. Li, L. Sun, C. Zhang et al., Polyanthraquinone-triazine-a promising anode material for high-energy lithium-ion batteries. ACS Appl. Mater. Interfaces 10(43), 37023–37030 (2018). https://doi.org/10.1021/acsami.8b12888
Y. Huang, K. Li, J. Liu, X. Zhong, X. Duan et al., Three-dimensional graphene/polyimide composite-derived flexible high-performance organic cathode for rechargeable lithium and sodium batteries. J. Mater. Chem. A 5(6), 2710–2716 (2017). https://doi.org/10.1039/c6ta09754e
A. Ahmad, H. Wu, Y. Guo, Q. Meng, Y. Meng et al., A graphene supported polyimide nanocomposite as a high performance organic cathode material for lithium ion batteries. RSC Adv. 6(40), 33287–33294 (2016). https://doi.org/10.1039/c5ra27471k
Z. Xiao, G. Xiang, Q. Zhang, Y. Wang, Y.J.E. Yang et al., Boosting lithium storage in graphene-sandwiched cathodes containing multi-carbonyl polyquinoneimine nanosheets. Energy Environ. Mater. (2022). https://doi.org/10.1002/eem2.12399
Q. Ban, Y. Liu, P. Liu, Y. Li, Y. Qin et al., Hierarchically nanostructured carbon nanotube/polyimide/mesoporous Fe2O3 nanocomposite for organic-inorganic lithium-ion battery anode. Micropor. Mesopor. Mater. 335, 111803–111812 (2022). https://doi.org/10.1016/j.micromeso.2022.111803
G. Zhang, Z. Xu, P. Liu, Y. Su, T. Huang et al., A facile in-situ polymerization strategy towards polyimide/carbon black composites as high performance lithium ion battery cathodes. Electrochim. Acta 260, 598–605 (2018). https://doi.org/10.1016/j.electacta.2017.12.075
G. Zha, C. Ouyang, S. Yin, K. Yao, S. Agarwal et al., High cycling stability of the LiNi0.8CO0.1Mn0.1O2 cathode via surface modification with polyimide/multi-walled carbon nanotubes composite coating. Small 17(47), 2102981 (2021). https://doi.org/10.1002/smll.202102981
J.-M. Kim, Y. Xu, M.H. Engelhard, J. Hu, H.-S. Lim et al., Facile dual-protection layer and advanced electrolyte enhancing performances of cobalt-free/nickel-rich cathodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 14(15), 17405–17414 (2022). https://doi.org/10.1021/acsami.2c01694
Y. Li, L. Lv, W. Huang, Y. Zhu, In situ polymerized and imidized Si@ polyimide microcapsules with flexible solid-electrolyte interphase and enhanced electrochemical activity for Li-storage. ChemElectroChem 9(2), e202101409 (2022). https://doi.org/10.1002/celc.202101409
Y. Wang, K. Qi, N. Dong, B. Liu, G. Tian, LiNi0.8CO0.1Mn0.1O2 Surface modification enabling 4. 7 v nickel-rich layered cathode with superior long-term cyclability via novel functional polyimide binders. J. Power Sources 545, 231927–231938 (2022). https://doi.org/10.1016/j.jpowsour.2022.231927
Y. Xu, Y. Wang, N. Dong, C. Pu, B. Liu et al., Novel polyimide binder for achieving high-rate capability and long-term cycling stability of LiNi0.8CO0.1Mn0.1O2 cathode via constructing polar and micro-branched crosslinking network structure. J. Energy Chem. 76, 19–31 (2023). https://doi.org/10.1016/j.jechem.2022.09.008
Y. Wang, N. Dong, B. Liu, K. Qi, G. Tian et al., Enhanced electrochemical performance of the LiNi0.8CO0.1Mn0.1O2 cathode via in-situ nanoscale surface modification with poly (imide-siloxane) binder. Chem. Eng. J. 450, 137959–137969 (2022). https://doi.org/10.1016/j.cej.2022.137959
Acknowledgements
We are grateful for the financial support provided by the National Natural Science Foundation of China (nos. U21A20170 [X. He], 22279070 [L. Wang], and 52206263 [Y. Song]) and the Ministry of Science and Technology of China (no. 2019YFA0705703 [L. Wang]). We want to thank the “Explorer 100” cluster system of Tsinghua National Laboratory for Information Science and Technology for their facility support.
Funding
Open access funding provided by Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Contributions
MZ contributed to draft writing and review. YS was involved in writing, reviewing, funding acquisition, and editing. HX, LW, and Xiangming He were involved in supervision, conceptualization, writing, reviewing, editing, and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, M., Wang, L., Xu, H. et al. Polyimides as Promising Materials for Lithium-Ion Batteries: A Review. Nano-Micro Lett. 15, 135 (2023). https://doi.org/10.1007/s40820-023-01104-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-023-01104-7