Abstract
Purpose
Improved methods for obtaining connective tissue structures in cardiac surgery are actively developed over the world. An important aspect of this research pertains to long-term wet storage of cardiovascular biomaterial that optimally preserves the initial morphological characteristics.
Methods
Native and decellularized porcine aortas were stored for 50 days in several biocidal solutions (complex alcohol solution, a mixture of ethanol and glycerol, a mixture of antibiotics), and subsequently implanted in animals and studied histologically. The aortic specimens were implanted subcutaneously into 15 adult WAG rats. The samples were retrieved after 3 months and evaluated for calcium content using a quantitative spectroanalytical procedure. Aortic specimens were also orthotopically implanted into the vascular bed of 3 minipigs, harvested after 6 months, and histologically evaluated.
Results
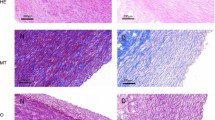
Atomic absorption spectroscopy showed that decellularized materials accumulated 4.8–9.2 times less calcium, depending on the storage solution used. Morphological and morphometrical analysis of orthotopically implanted aortic fragments in minipigs showed better preservation of decellularized material that had been stored in a complex alcohol solution and mixture of antibiotics.
Conclusion
Functional and histological evidence was obtained demonstrating long-term storage without freezing of o aortic specimens without alteration of their biophysical properties and structure. The developed technique will be used in the future for the fabrication of vascular prostheses and heart valves.






Similar content being viewed by others
References
Lisy, M., Kalender, G., Schenke-Layland, K., Brockbank, K. G., Biermann, A., & Stock, U. A. (2017). Allograft Heart Valves: current aspects and future applications. Biopreservation and Biobanking, 15(2), 148–157. https://doi.org/10.1089/bio.2016.0070.
Fioretta, E. S., von Boehmer, L., Motta, S. E., Lintas, V., Hoerstrup, S. P., & Emmert, M. Y. (2019). Cardiovascular tissue engineering: From basic science to clinical application. Experimental Gerontology, 117, 1–12. https://doi.org/10.1016/j.exger.2018.03.022
VeDepo, M. C., Detamore, M. S., Hopkins, R. A., & Converse, G. L. (2017). Recellularization of decellularized heart valves: Progress toward the tissue-engineered heart valve. Journal of Tissue Engineering, 8, 2041731417726327. https://doi.org/10.1177/2041731417726327.
Zhuravleva, I. Y., Nichay, N. R., Kulyabin, Y. Y., Timchenko, T. P., Korobeinikov, A. A., Polienko, Y. F., Shatskaya, S. S., Kuznetsova, E. V., Voitov, A. V., Bogachev-Prokophiev, A. V., & Karaskov, A. M. (2018). In search of the best xenogeneic material for a paediatric conduit: an experimental study. Interactive Cardiovascular And Thoracic Surgery, 26, 738–744. https://doi.org/10.1093/icvts/ivx445.
Lee, J., Chan, M. C., James, C., & Lantis, J. C. (2020). Cryopreserved allograft use in vascular surgery. Surgical Technology International, 37, 237–243.
Britikov, D. V., Lauk-Dubitski, S., Serov, R., & Hugaev, G. (2019). Morphological evaluation of a new technique for cryopreservation of valvular and vascular allografts. Annals of Surgery, 24(1), 16–23. https://doi.org/10.24022/1560-9502-2019-24-1-16-23(In Russ.).
Wollmann, L. C., Suss, P. H., Kraft, L., Ribeiro, V. S., Noronha, L., da Costa, F. D. A., & Tuon, F. F. (2020). Histological and biomechanical characteristics of human decellularized allograft heart valves after eighteen months of storage in saline solution. Biopreservation and Biobanking, 18, 90–101. https://doi.org/10.1089/bio.2019.0106.
Kraft, L., Ribeiro, V. S. T., & de Nazareno Wollmann, L. C. F. (2020). Determination of antibiotics and detergent residues in decellularized tissue-engineered heart valves using LC–MS/MS. Cell and Tissue Banking, 21, 573–584. https://doi.org/10.1007/s10561-020-09856-x.
VeDepo, M. C., Buse, E. E., Quinn, R. W., Williams, T. D., Detamore, M. S., Hopkins, R. A., & Converse, G. L. (2017). Species-specific effects of aortic valve decellularization. Acta Biomaterialia, 50, 249–258. https://doi.org/10.1016/j.actbio.2017.01.008.
Keane, T. J., Londono, R., Turner, N. J., & Badylak, S. F. (2012). Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials, 33, 1771–1781. https://doi.org/10.1016/j.biomaterials.2011.10.054.
Vasiliyeva, M. B., Kuznetsova, E. V., Rusakova, Y. L., Chepeleva, Е. V., Dokuchaeva, A. A., Bukreeva, L. N., & Sergeevichev, D. S. (2020). Effects of biocidal solutions and wet tissue treatment on the biological properties of porcine aortic wall. Complex Issues of Cardiovasc Diseases, 9, 63–73. https://doi.org/10.17802/2306-1278-2020-9-1-63-73(In Russ.).
Lichtenberg, A., Tudorache, I., Cebotari, S., Ringes-Lichtenberg, S., & Sturz, G. (2006). In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials, 27, 4221–4229. https://doi.org/10.1016/j.biomaterials.200603.047.
Krasilnikova, A., Sergeevichev, D., Fomenko, V., Korobeynikov, A., Vasiliyeva, M., Yunoshev, A., Karaskov, A., & Pokushalov, E. (2018). Globular chitosan treatment of bovine jugular veins: evidence of anticalcification efficacy in the subcutane-ous rat model. Cardiovascular Pathology, 32, 1–7. https://doi.org/10.1016/j.carpath.2017.08.003.
Zhuravleva, I. Y. (2016). Biocidal composition for aseptic storage of prosthetic material from animal tissue. Patent for invention RU 2580621C1. Bull, 10, 1–10.
Shavandi, A., Bekhit, A. E. A., Saeedi, P., Izadifar, Z., Bekhit, A. A., & Khademhosseini, A. (2018). Polyphenol uses in biomaterials engineering. Biomaterials, 167, 91–106.
Theodoridis, K., Müller, J., Ramm, R., Findeisen, K., Andrée, B., Korossis, S., Haverich, A., & Hilfiker, A. (2016). Effects of combined cryopreservation and decellularization on the biomechanical, structural and biochemical properties of porcine pulmonary heart valves. Acta Biomaterialia, 43, 71–77. https://doi.org/10.1016/j.actbio.2016.07.013.
Collatusso, C., Roderjan, J. G., de Noronha, L., Klosowski, A., Suss, P. H., Guarita-Souza, L. C., & Costa, F. D. A. D. (2019). Decellularization as a method to reduce calcification in bovine pericardium bioprosthetic valves. Interactive Cardiovascular and Thoracic Surgery, 29, 302–311. https://doi.org/10.1093/icvts/ivz041.
Huyan, Y., Chang, Y., & Song, J. (2021). Application of homograft valved conduit in cardiac surgery. Frontiers in Cardiovascular Medicine, 8, 740871. https://doi.org/10.3389/fcvm.2021.740871.
Dréno, B., Zuberbier, T., Gelmetti, C., Gontijo, G., & Marinovich, M. (2019). Safety review of phenoxyethanol when used as a preservative in cosmetics. Journal of the European Academy of Dermatology and Venereology, 33, 15–24. https://doi.org/10.1111/jdv.15944.
Yeo, S. Y., Hamzan, M. I., Sulaiman, W., & Halim, W. A., A.S (2021). The cost-effectiveness of maintaining an inhouse glycerol-preserved skin bank. Burns : Journal Of The International Society for Burn Injuries, 48, 390–395. https://doi.org/10.1016/j.burns.2021.05.001.
Ziza, V., Canaud, L., Gandet, T., Molinari, N., Alonso, W., Chastan, R., Branchereau, P., & Picard, E. (2015). Outcomes of cold-stored venous allograft for below-knee bypasses in patients with critical limb ischemia. Journal of Vascular Surgery, 62, 974–983. https://doi.org/10.1016/j.jvs.2015.04.437.
Badria, A. F., Koutsoukos, P. G., & Mavrilas, D. (2020). Decellularized tissue-engineered heart valves calcification: what do animal and clinical studies tell us? Journal of Materials Science: Materials in Medicine, 31, 132. https://doi.org/10.1007/s10856-020-06462-x.
Gallyamov, M. O., Chaschin, I. S., Khokhlova, M. A., Grigorev, T. E., Bakuleva, N. P., & Lyutova, I. G. (2014). Collagen tissue treated with chitosan solutions in carbonic acid for improved biological prosthetic heart valves. Materials Science & Engineering C Materials for Biological Applications, 37, 127–140. https://doi.org/10.1016/j.msec.2014.01.017.
Khorramirouz, R., Go, J. L., Noble, C., Jana, S., Maxson, E., Lerman, A., & Young, M. D. (2018). A novel surgical technique for a rat subcutaneous implantation of a tissue engineered scaffold. Acta Histochemica, 120(3), 282–291. https://doi.org/10.1016/j.acthis.2018.02.010.
Cramer, M., Chang, J., Li, H., Serrero, A., El-Kurdi, M., Cox, M., Schoen, F. J., & Badylak, S. F. (2022). Tissue response, macrophage phenotype, and intrinsic calcification induced by cardiovascular biomaterials: can clinical regenerative potential be predicted in a rat subcutaneous implant model? Journal of Biomedical Materials Research Part A, 110, 245–256. https://doi.org/10.1002/jbm.a.37280.
Simionescu, D. T. (2004). Prevention of calcification in bioprosthetic heart valves: Challenges and perspectives. Expert Opinion on Biological Therapy, 4(12), 1971–1985. https://doi.org/10.1517/14712598.4.12.1971.
McGregor, C. G. A., Carpentier, A., Lila, N., Logan, J. S., & Byrne, G. W. (2011). Cardiac xenotransplantation technology provides mate-rials for improved bioprosthetic heart valves. The Journal of Thoracic and Cardiovascular Surgery, 141, 269–275. https://doi.org/10.1016/j.jtcvs.2010.08.064.
Kluin, J., et al. (2017). In situ heart valve tissue engineering using a bioresorbable elastomeric implant – from material design to 12 months follow-up in sheep. Biomaterials, 125, 101–117. https://doi.org/10.1016/j.biomaterials.2017.02.007.
Meuris, B., Ozaki, S., Herijgers, P., Verbeken, E., & Flameng, W. (2003). Bioprosthetic tissue calcification: influence of blood contact and arterial pressure. An experimental study in rats and sheep. Journal of Heart Valve Diseases, 12(3), 392–399.
Connolly, J. M., Bakay, M. A., Alferiev, I. S., Gorman, R. C., & Gorman, J. H. (2011). Triglycidylamine cross-linking combined with ethanol inhibits bioprosthetic heart valve calcification. The Annals of Thoracic Surgery, 92(3), 858–865. https://doi.org/10.1016/j.athoracsur.2011.04.104.
Magsumov, T., Ziyinga, L., & Sedov, I. (2020). Comparative study of the protein denaturing ability of different organic cosolvents. International Journal of Biological Macromolecules, 160, 880–888. https://doi.org/10.1016/j.ijbiomac.2020.05.260.
Acknowledgements
This work was carried out within the state assignment of Ministry of Health of Russian Federation (theme # 121031300224-1).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DS, MV and EK. MZ, YR. and EC. conducted animal experiments. DS analysed data and wrote the manuscript. IZ helped material preparation and analysis with constructive discussions All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests regarding this manuscript.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local ethical committee of NMRC named after academician E.N. Meshalkin of the Ministry of Health of the Russian Federation (approval date 26 Dec 2014, protocol 45).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sergeevichev, D., Vasiliyeva, M., Kuznetsova, E. et al. Morphological Post-implantation Features of Aortic Conduits After Long-term wet Storage. J. Med. Biol. Eng. 43, 185–194 (2023). https://doi.org/10.1007/s40846-023-00784-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-023-00784-1