Abstract
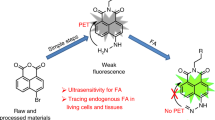
Formaldehyde (FA, a typical reactive carbonyl species) is a well-known environmental pollutant and a disease-related biomarker, making its sensitive and selective detection significant. Fluorescent probes have been explored for FA perception in environment, intracellular media and in vivo. In this review, we majorly conclude the recently represented fluorescence FA analysis based on small molecule probes. The general FA sensing mechanisms are first introduced. Regarding the FA detection in various environments, sensing tactics and performances are discussed in order of natural environment, living cells and in vivo. In the end, this review discusses the challenges and future trends of FA detection based on fluorescent probes.

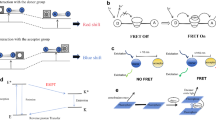
Copyright 2018, Royal Society of Chemistry. b Illustration of AIEgen–mediated fluorescence turn-on mechanism for FA detection. Reprinted with permission from ref. [25]. Copyright 2018, American Chemical Society

Copyright 2016, Wiley–VCH. b Response mechanism of two-photon FRET-based ratiometric fluorescent sensor toward FA. Reprinted with permission from ref. [30]. Copyright 2020, Elsevier

Copyright 2019, Royal Society of Chemistry. b Diagram of FA-induced ring-opening of azacyclo probe. Reprinted with permission from ref. [45]. Copyright 2021, Royal Society of Chemistry

Copyright 2018, American Chemical Society. b The fluorescence spectra of the TF-FA loaded fabric and cotton test substrates in the absence and presence of FA, excited at 305 nm. Inset images are the corresponding photographs of fabric (left) and cotton (right) test substrates in the absence and presence of FA. Reprinted with permission from ref. [53]. Copyright 2019, Elsevier

Copyright 2021, Royal Society of Chemistry. b Confocal fluorescence imaging of HeLa (a1–a3), L929 (b1–b2) and HepG-2 (c1–c2) cells incubated with 5 μmol/L NFP-G. Reprinted with permission from ref. [63]. Copyright 2021, Elsevier

Copyright 2016, Wiley–VCH. b Ratiometric imaging of FA in different mouse organ tissues. Reprinted with permission from ref. [68]. Copyright 2017, American Chemical Society. c Schematic illustration of “dual-key and lock” Ru-FA probe for lysosomal FA sensing. Reprinted with permission from ref. [69]. Copyright 2019, American Chemical Society

Copyright 2017, Royal Society of Chemistry. b Confocal microscopy images of Arabidopsis thaliana root tip tissues after BT-1 incubation without (up) and with (bottom) exogenous FA stimulation. Reprinted with permission from ref. [70]. Copyright 2018, Elsevier
Similar content being viewed by others
References
Birlouez-Aragon I, Morales F, Fogliano V, Pain JP. The health and technological implications of a better control of neoformed contaminants by the food industry. Pathol Biol. 2010;58(3):232–8.
Casset A, Marchand C, Le Calvé S, Mirabel P, de Blay F. Human exposure chamber for known formaldehyde levels: generation and validation. Indoor Built Environ. 2005;14(2):173–82.
Reingruber H, Pontel LB. Formaldehyde metabolism and its impact on human health. Curr Opin Toxicol. 2018;9:28–34.
Burgos-Barragan G, Wit N, Meiser J, Dingler FA, Pietzke M, Mulderrig L, Pontel LB, Rosado IV, Brewer TF, Cordell RL, Monks PS, Chang CJ, Vazquez A, Patel KJ. Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature. 2017;548(7669):549–54.
Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: a global review. Cancer Epidemiol. 2016;44:203–21.
Jiang X, Li C, Chi Y, Yan J. TG-FTIR study on urea-formaldehyde resin residue during pyrolysis and combustion. J Hazard Mater. 2010;173(1–3):205–10.
Duan H, Deng W, Gan Z, Li D, Li D. SERS-based chip for discrimination of formaldehyde and acetaldehyde in aqueous solution using silver reduction. Microchim Acta. 2019;186(3):175–86.
Yuan ZC, Hu B. Mass spectrometry-based human breath analysis: towards COVID-19 diagnosis and research. J Anal Test. 2021;5(4):287–97.
Tang Y, Ma Y, Yin J, Lin W. Strategies for designing organic fluorescent probes for biological imaging of reactive carbonyl species. Chem Soc Rev. 2019;48(15):4036–48.
Li HY, Zhao SN, Zang SQ, Li J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem Soc Rev. 2020;49(17):6364–401.
Xu Z, Chen J, Hu LL, Tan Y, Liu SH, Yin J. Recent advances in formaldehyde-responsive fluorescent probes. Chin Chem Lett. 2017;28(10):1935–42.
Li HJ, Sun X, Xue F, Ou N, Sun BW, Qian DJ, Chen M, Wang D, Yang J, Wang X. Redox induced fluorescence on–off switching based on nitrogen enriched graphene quantum dots for formaldehyde detection and bioimaging. ACS Sustain Chem Eng. 2018;6(2):1708–16.
Nandi S, Sharma E, Trivedi V, Biswas S. Metal-organic framework showing selective and sensitive detection of exogenous and endogenous formaldehyde. Inorg Chem. 2018;57(24):15149–57.
Burris AJ, Tran K, Cheng Q. Tunable enhancement of a graphene/polyaniline/poly(ethylene oxide) composite electrospun nanofiber gas sensor. J Anal Test. 2017;1(2):12–22.
Liu X, Li N, Li M, Chen H, Zhang N, Wang Y, Zheng K. Recent progress in fluorescent probes for detection of carbonyl species: Formaldehyde, carbon monoxide and phosgene. Coord Chem Rev. 2020;404:213109.
Cheng D, Xu W, Gong X, Yuan L, Zhang XB. Design strategy of fluorescent probes for live drug-induced acute liver injury imaging. Acc Chem Res. 2021;54(2):403–15.
Jiang WL, Wang WX, Mao GJ, Yan L, Du Y, Li Y, Li CY. Construction of nir and ratiometric fluorescent probe for monitoring carbon monoxide under oxidative stress in zebrafish. Anal Chem. 2021;93(4):2510–8.
She ZP, Wang WX, Jiang WL, Wang ZQ, Mao GJ, Fei J, Li Y, Li CY. Accurate fluorescence diagnosis of cancer based on sequential detection of hydrogen sulfide and pH. Anal Chem. 2021;93(34):11826–35.
Li K, Ren TB, Huan S, Yuan L, Zhang XB. Progress and perspective of solid-state organic fluorophores for biomedical applications. J Am Chem Soc. 2021;143(50):21143–60.
Bruemmer KJ, Brewer TF, Chang CJ. Fluorescent probes for imaging formaldehyde in biological systems. Curr Opin Chem Biol. 2017;39(17):17–23.
Liu X, Manzur C, Novoa N, Celedón S, Carrillo D, Hamon JR. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord Chem Rev. 2018;357:144–72.
Song H, Rajendiran S, Kim N, Jeong SK, Koo E, Park G, Thangadurai TD, Yoon S. A tailor designed fluorescent ‘turn-on’ sensor of formaldehyde based on the BODIPY motif. Tetrahedron Lett. 2012;53(37):4913–6.
Song X, Han X, Yu F, Zhang J, Chen L, Lv C. A reversible fluorescent probe based on C=N isomerization for the selective detection of formaldehyde in living cells and in vivo. Analyst. 2018;143(2):429–39.
Wen X, Yan L, Fan Z. One-step construction of a novel AIE probe based on diaminomaleonitrile and its application in double-detection of hypochlorites and formaldehyde gas. New J Chem. 2021;45(18):8155–65.
Chen W, Han J, Wang X, Liu X, Liu F, Wang F, Yu RQ, Jiang JH. Aggregation-induced emission-based fluorescence probe for fast and sensitive imaging of formaldehyde in living cells. ACS Omega. 2018;3(10):14417–22.
Martínez-Aquino C, Costero AM, Gil S, Gaviña P. A new environmentally-friendly colorimetric probe for formaldehyde gas detection under real conditions. Molecules. 2018;23(10):2646–54.
Li P, Zhang D, Zhang Y, Lu W, Wang W, Chen T. Ultrafast and efficient detection of formaldehyde in aqueous solutions using chitosan-based fluorescent polymers. ACS Sens. 2018;3(11):2394–401.
Tang Y, Kong X, Xu A, Dong B, Lin W. Development of a two-photon fluorescent probe for imaging of endogenous formaldehyde in living tissues. Angew Chem Int Ed. 2016;55(10):3356–9.
Nasirian A, Tikum AF, Fortibui MM, Lee S, Kim J. Napthalimide-based fluorescent probe for selective and sensitive sensing of formaldehyde and biological applications. Dyes Pigm. 2021;188:109156.
Yuan G, Ding H, Peng L, Zhou L, Lin Q. A novel fluorescent probe for ratiometric detection of formaldehyde in real food samples, living tissues and zebrafish. Food Chem. 2020;331:127221.
Wei L, Wang CJ. Recent advances in catalytic asymmetric aza-Cope rearrangement. Chem Commun. 2021;57(81):10469–83.
Rueping M, Antonchick AP. Catalytic asymmetric aminoallylation of aldehydes: a catalytic enantioselective Aza-Cope rearrangement. Angew Chem Int Ed. 2008;47(52):10090–3.
Fiedler D, Bergman RG, Raymond KN. Supramolecular catalysis of a unimolecular transformation: Aza-Cope rearrangement within a self-assembled host. Angew Chem Int Ed. 2004;43(48):6748–51.
Brewer TF, Burgos-Barragan G, Wit N, Patel KJ, Chang CJ. A 2-aza-Cope reactivity-based platform for ratiometric fluorescence imaging of formaldehyde in living cells. Chem Sci. 2017;8(5):4073–81.
Mc Cormack MP, Shalumova T, Tanski JM, Waters SP. Development of a 2-Aza-Cope-[3+2] dipolar cycloaddition strategy for the synthesis of quaternary proline scaffolds. Org Lett. 2010;12(17):3906–9.
Brewer TF, Chang CJ. An Aza-Cope reactivity-based fluorescent probe for imaging formaldehyde in living cells. J Am Chem Soc. 2015;137(34):10886.
Li Z, Xu Y, Zhu H, Qian Y. Imaging of formaldehyde in plants with a ratiometric fluorescent probe. Chem Sci. 2017;8(8):5616–21.
Gu J, Li X, Zhou G, Liu W, Gao J, Wang Q. A novel self-calibrating strategy for real time monitoring of formaldehyde both in solution and solid phase. J Hazard Mater. 2020;386:121883.
Yang X, He L, Xu K, Yang Y, Lin W. The development of an ICT-based formaldehyde-responsive fluorescence turn-on probe with a high signal-to-noise ratio. New J Chem. 2018;42(15):12361–4.
Zhang Y, Du Y, Li M, Zhang D, Xiang Z, Peng T. Activity-based genetically encoded fluorescent and luminescent probes for detecting formaldehyde in living cells. Angew Chem Int Ed. 2020;59(38):16352–6.
Du Y, Zhang Y, Huang M, Wang S, Wang J, Liao K, Wu X, Zhou Q, Zhang X, Wu YD, Peng T. Systematic investigation of the aza-Cope reaction for fluorescence imaging of formaldehyde in vitro and in vivo. Chem Sci. 2021;12(41):13857–69.
Ma Y, Gao W, Zhu L, Zhao Y, Lin W. Development of a unique reversible fluorescent probe for tracking endogenous sulfur dioxide and formaldehyde fluctuation in vivo. Chem Commun. 2019;55(75):11263–6.
Wang M, Liu Q, Sun X, Zheng S, Ma Y, Wang Y, Yan M, Lu Z, Fan C, Lin W. Ratiometric and reversible detection of endogenous SO2 and HCHO in living cells and mice by a near-infrared and dual-emission fluorescent probe. Sens Actuators B. 2021;335:129649.
Tan L, Ding H, Chanmungkalakul S, Peng L, Yuan G, Yang Q, Liu X, Zhou L. A smart TP-FRET-based ratiometric fluorescent sensor for bisulfite/formaldehyde detection and its imaging application. Sens Actuators B. 2021;345:130331.
Bi A, Liu M, Huang S, Zheng F, Ding J, Wu J, Tang G, Zeng W. Construction and theoretical insights into the ESIPT fluorescent probe for imaging formaldehyde in vitro and in vivo. Chem Commun. 2021;57(28):3496–9.
Ge H, Liu G, Yin R, Sun Z, Chen H, Yu L, Su P, Sun M, Alamry KA, Marwani HM, Wang S. An aldimine condensation reaction based fluorescence enhancement probe for detection of gaseous formaldehyde. Microchem J. 2020;156:104793.
Zhao X, Ji C, Ma L, Wu Z, Cheng W, Yin M. An aggregation-induced emission-based “turn-on” fluorescent probe for facile detection of gaseous formaldehyde. ACS Sens. 2018;3(10):2112–7.
Liu T, Yang L, Zhang J, Liu K, Ding L, Peng H, Belfield KD, Fang Y. Squaraine-hydrazine adducts for fast and colorimetric detection of aldehydes in aqueous media. Sens Actuators B. 2019;292:88–93.
Wang Y, Sun X, Han Q, James TD, Wang X. Highly sensitive and selective water-soluble fluorescent probe for the detection of formaldehyde in leather products. Dyes Pigm. 2021;188:109175.
Xin F, Tian Y, Jing J, Zhang X. A two-photon fluorescent probe for imaging of endogenous formaldehyde in HeLa cells and quantitative detection of basal formaldehyde in milk samples. Anal Methods. 2019;11(23):2969–75.
Zhou Y, Yan J, Zhang N, Li D, Xiao S, Zheng K. A ratiometric fluorescent probe for formaldehyde in aqueous solution, serum and air using Aza-Cope reaction. Sens Actuators B. 2018;258:156–62.
Taihong Liu RM, Haonan P, Jing L, Liping D, Yu F. Adlayer chemistry on film-based fluorescent gas sensors. Acta Phys-Chim Sin. 2020;36(10):1908025.
Zhai B, Zhang Y, Hu Z, He J, Liu J, Gao C, Li W. A ratiometric fluorescent probe for the detection of formaldehyde in aqueous solution and air via Aza-Cope reaction. Dyes Pigm. 2019;171:107743.
Chen J, Chen K, Han B, Xue Y, Chen W, Gao Z, Hou X. A novel single-fluorophore-based ratiometric fluorescent probe for detection of formaldehyde in air. Tetrahedron. 2020;76(50):131681.
Yu R, Lai Y, Hartwell HJ, Moeller BC, Doyle-Eisele M, Kracko D, Bodnar WM, Starr TB, Swenberg JA. Formation, accumulation, and hydrolysis of endogenous and exogenous formaldehyde-induced DNA damage. Toxicol Sci. 2015;146(1):170–82.
Yuan W, Zhong X, Han Q, Jiang Y, Shen J, Wang B. A novel formaldehyde fluorescent probe based on 1, 8-naphthalimide derivative and its application in living cell. J Photochem Photobiol A. 2020;400:112701.
Gu L, Tang Y, Lin W. A new highly selective fluorescence probe for the imaging of endogenous formaldehyde in living cells. Tetrahedron. 2021;78:131808.
Bi A, Gao T, Cao X, Dong J, Liu M, Ding N, Liao W, Zeng W. A novel naphthalimide-based probe for ultrafast, highly selective and sensitive detection of formaldehyde. Sens Actuators B. 2018;255:3292–7.
Xu J, Zhang Y, Zeng L, Liu J, Kinsella JM, Sheng R. A simple naphthalene-based fluorescent probe for high selective detection of formaldehyde in toffees and HeLa cells via aza-Cope reaction. Talanta. 2016;160:645–52.
He L, Yang X, Liu Y, Kong X, Lin W. A ratiometric fluorescent formaldehyde probe for bioimaging applications. Chem Commun. 2016;52(21):4029–32.
Zhang D, Liu D, Li M, Yang Y, Wang Y, Yin H, Liu J, Jia B, Wu X. A simple pyrene-based fluorescent probe for highly selective detection of formaldehyde and its application in live-cell imaging. Anal Chim Acta. 2018;1033:180–4.
Sheng W, Zhang X, Yu M, Jin M, Li N, Sun C, Wang L, Xia Q, Li X, Zhang Y, Zhu B, Liu K. A novel cell membrane-targeting fluorescent probe for imaging endogenous/exogenous formaldehyde in live cells and zebrafish. Analyst. 2021;146(24):7554–62.
Zhou L, Cui J, Yu Z, Zou D, Zhang W, Qian J. A β-d-galactose-guided fluorescent probe for selectively bioimaging endogenous formaldehyde in living HepG-2 cells. Sens Actuators B. 2021;332:129494.
Liang XG, Chen B, Shao LX, Cheng J, Huang MZ, Chen Y, Hu YZ, Han YF, Han F, Li X. A fluorogenic probe for ultrafast and reversible detection of formaldehyde in neurovascular tissues. Theranostics. 2017;7(8):2305–13.
Tang Y, Zhao Y, Lin W. Preparation of robust fluorescent probes for tracking endogenous formaldehyde in living cells and mouse tissue slices. Nat Protoc. 2020;15(10):3499–526.
Lee YH, Tang Y, Verwilst P, Lin W, Kim JS. A biotin-guided formaldehyde sensor selectively detecting endogenous concentrations in cancerous cells and tissues. Chem Commun. 2016;52(75):11247–50.
Chen J, Shao C, Wang X, Gu J, Zhu HL, Qian Y. Imaging of formaldehyde fluxes in epileptic brains with a two-photon fluorescence probe. Chem Commun. 2020;56(27):3871–4.
Singha S, Jun YW, Bae J, Ahn KH. Ratiometric imaging of tissue by two-photon microscopy: observation of a high level of formaldehyde around mouse intestinal crypts. Anal Chem. 2017;89(6):3724–31.
Liu C, Zhang R, Zhang W, Liu J, Wang YL, Du Z, Song B, Xu ZP, Yuan J. A “dual-key-and-lock” ruthenium complex probe for lysosomal formaldehyde in cancer cells and tumors. J Am Chem Soc. 2019;141(21):8462–72.
Wu Y, Zheng Z, Wen J, Li H, Sun S, Xu Y. Imaging of formaldehyde in live cells and plants utilizing small molecular probes with large stokes shifts. Sens Actuators B. 2018;260:937–44.
Cao Y, Teng Z, Zhang J, Cao T, Qian J, Wang J, Qin W, Guo H. A fluorescent probe for distinguish detection of formaldehyde and acetaldehyde. Sens Actuators B. 2020;320:128354.
Sun Y, Lu F, Yang H, Ding C, Yuan Z, Lu C. Fluorescent sensor array for separation-free dopamine analogue discrimination via polyethyleneimine-mediated self-polymerization reaction. Nanoscale. 2019;11(27):12889–97.
Yang H, Lu F, Sun Y, Yuan Z, Lu C. Fluorescent gold nanocluster-based sensor array for nitrophenol isomer discrimination via an integration of host–guest interaction and inner filter effect. Anal Chem. 2018;90(21):12846–53.
Yuan Z, Du Y, Tseng YT, Peng M, Cai N, He Y, Chang HT, Yeung ES. Fluorescent gold nanodots based sensor array for proteins discrimination. Anal Chem. 2015;87(8):4253–9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22074005), the Natural Science Foundation of Beijing Municipality (2202038), the Open Research Fund Program of Beijing Key Lab of Plant Resource Research and Development, Beijing Technology and Business University (PRRD-2021-YB6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zheng, JJ., Liu, WC., Lu, FN. et al. Recent Progress in Fluorescent Formaldehyde Detection Using Small Molecule Probes. J. Anal. Test. 6, 204–215 (2022). https://doi.org/10.1007/s41664-022-00220-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-022-00220-4