Abstract
Aim
There is currently limited evidence on the costs associated with late preterm pre-eclampsia beyond antenatal care and post-natal discharge from hospital. The aim of this analysis is to evaluate the 24-month cost-utility of planned delivery for women with late preterm pre-eclampsia at 34+0–36+6 weeks’ gestation compared to expectant management from an English National Health Service perspective using participant-level data from the PHOENIX trial.
Methods
Women between 34+0 and 36+6 weeks’ gestation in 46 maternity units in England and Wales were individually randomised to planned delivery or expectant management. Resource use was collected from hospital records between randomisation and primary hospital discharge following birth. Women were followed up at 6 months and 24 months following birth and self-reported resource use for themselves and their infant(s) covering the previous 6 months. Women completed the EQ-5D 5L at randomisation and follow-up.
Results
A total of 450 women were randomised to planned delivery, 451 to expectant management: 187 and 170 women, respectively, had complete data at 24 months. Planned delivery resulted in a significantly lower mean cost per woman and infant(s) over 24 months (− £2711, 95% confidence interval (CI) − 4840 to − 637), with a mean incremental difference in QALYs of 0.019 (95% CI − 0.039 to 0.063). Short-term and 24-month infant costs were not significantly different between the intervention arms. There is a 99% probability that planned delivery is cost-effective at all thresholds below £37,000 per QALY gained.
Conclusion
There is a high probability that planned delivery is cost-effective compared to expectant management. These results need to be considered alongside clinical outcomes and in the wider context of maternity care.
Trial registration: ISRCTN registry ISRCTN01879376. Registered 25 November 2013.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For women who are at least 34 weeks’ and fewer than 37 weeks’ gestation with a diagnosis of pre-eclampsia, at 24 months there is no evidence that planned delivery, initiation of delivery within 48 h, results in additional costs to the health service compared to expectant management. There is no difference in health-related quality of life for the women. |
Given the evidence that planned delivery results in cost-savings to the health service for antenatal care, there is a high-probability that planned delivery is cost-effective compared to expectant management. |
1 Introduction
Pre-eclampsia complicates 2.8% of singleton pregnancies in high-income countries [1], and can be associated with severe complications for the woman and infant; the disease is still a major cause of maternal death worldwide [2]. In the USA in 2012, pre-eclampsia cost US$2.18 billion within the first 12 months of birth [3]. A large proportion of the cost is to the healthcare system; this is partially due to the high cost of antenatal care as a result of frequent foetal and maternal surveillance [4] in addition to the costs associated with increased rates of neonatal admission (compared to pregnancies uncomplicated by pre-eclampsia), due to the lower gestational age and a greater number of maternal and infant adverse events [5, 6].
For women with pre-eclampsia, delivery is currently recommended at 37 weeks’ gestation or sooner if clinical need should arise [7]. For women with pre-eclampsia between 34+0 and 36+6 weeks’ gestation, there is limited evidence to guide decisions around delivery, with a need to balance any short- and long-term benefits and risks. Earlier delivery may benefit both woman and infant, particularly by halting the disease process itself that can result in foetal growth restriction, but may have an uncertain impact on neurodevelopmental outcomes. Results from the analysis of short-term outcomes from the PHOENIX trial showed that planned delivery at 34+0 to 36+6 weeks’ gestation significantly reduced maternal adverse outcomes, but was accompanied by an increase in admissions to a neonatal unit (NNU) [8]. However, the increase in neonatal admissions did not override the additional costs associated with the increased antenatal care (and complications) for women randomised to expectant management, with planned delivery resulting in overall cost savings. This analysis though did not account for potential longer-term impact of earlier delivery on costs and did not include any assessment of health-related quality of life for the woman or infant.
The aim of this paper is to report on the 2-year mean incremental cost per quality-adjusted life year (QALY) gained of planned delivery at 34+0–36+6 weeks’ gestation compared to expectant management for women with late preterm pre-eclampsia recruited to the PHOENIX trial, from an English National Health Service (NHS) perspective.
2 Methods
2.1 Trial Summary
Forty-six maternity units across England and Wales enrolled pregnant women over 18 years of age between 34+0 and 36+6 weeks’ gestation with a diagnosis of pre-eclampsia or superimposed pre-eclampsia (women with chronic hypertension who go on to develop pre-eclampsia) to a parallel-group, non-masked, multicentre, randomised controlled trial. Women between 34+0 and 36+6 weeks’ gestation were randomised with a 1:1 allocation to planned initiation of delivery within 48 h of randomisation or to expectant management (usual care), with delivery at 37 weeks’ gestation or sooner as clinical needs dictated in accordance with the UK national guidelines [9]. The only trial exclusion criterion was a prior decision to deliver within the subsequent 48 h or that the woman was unable to provide informed consent. Pregnancies may have been singleton or dichorionic diamniotic twins and could include any foetal position. Women with co-morbidities including pre-existing hypertension and diabetes were not excluded. Women with a previous caesarean section were also eligible.
The aim of the study was to evaluate if planned delivery in women with pre-eclampsia between 34+0 and 36+6 weeks’ gestation reduces maternal adverse outcomes without substantial worsening of neonatal or infant outcomes, compared to the current practice of expectant management until 37 weeks’ gestation. The co-primary short-term maternal outcome was a composite of maternal morbidity at primary hospital discharge. The co-primary perinatal outcome was perinatal deaths (antenatal/intrapartum stillbirths or deaths within 7 days of delivery) or NNU admissions (physical separation of baby from the mother) prior to infant hospital discharge. The primary long-term infant outcome was neurodevelopmental assessment at 2 years of age (adjusted), using the Parent Report of Children’s Abilities-Revised (PARCA-R) parent report composite score. Full details of the primary clinical analyses are available in the trial protocol [10] and the paper describing the short-term clinical outcomes up to hospital discharge [8]. The trial was approved by the South Central-Hampshire B Research Ethics Committee (no. 13/SC/0645).
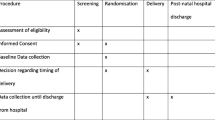
2.2 Procedures
Planned delivery consisted of initiation of delivery within 48 h of randomisation, to allow for administration of antenatal corticosteroids if deemed necessary by local clinicians. Induction of labour was commenced according to local protocol, with caesarean section undertaken only if an additional obstetric indication was present. Expectant management consisted of usual care, with close monitoring of the maternal and foetal condition until either 37 weeks’ gestation, or the development of severe features necessitating delivery. The management of pregnant women whilst in hospital and postnatal care was in accordance with the national guidelines for the management of hypertension in pregnancy [9].
2.3 Outcome Measures
Outcomes for the economic evaluation include short-term outcomes from randomisation until the primary maternal and infant discharge from hospital following birth (hereafter called ‘postnatal discharge’) and were collected from hospital clinical records and recorded on the web-based trial database. Women also completed the EQ-5D-5L [11] at randomisation. For 24-month outcomes, healthcare resource use including inpatient stays, emergency and outpatient attendances for the woman and infant(s) and health-related quality of life were collected from self-completed questionnaires at 6 months post-delivery and when the infant was 2 years of age (corrected for prematurity). The healthcare resource use questionnaire was a bespoke questionnaire, adapted from the Client Service Receipt Inventory [12].
Questionnaires were posted to all participants at these time points (or a link sent electronically), and participants were able to complete and return a paper copy via FREEPOST to the co-ordinating centre, or to complete an online version captured by the MedSciNet study database. For the EQ-5D-5L, women were directed to the EuroQoL online portal to complete the questionnaire, in line with EuroQoL requirements.
2.4 Economic Analysis
Analyses were pre-specified in a health economics analysis plan signed off prior to database lock (see the Electronic Supplementary Material [ESM]).
We calculated complete-case (women that were followed-up at that time point and completed the questionnaire for themselves and their infant) descriptive statistics for the percentage of women and infants and mean number of contacts for each type of resource use. Complete case means and standard deviations for costs were also calculated at each time point. Negative binomial regression, adjusting for minimisation factors (see below) was used to calculate the difference between arms and 95% confidence intervals (CIs) for maternal and infant bed days at 6 and 24 months.
Resource use was costed from the most recent version of NHS Reference Costs (2018/2019) [13] and the Personal and Social Service Resource Unit (PSSRU) [14] (see Table 1). Where possible a pre-eclampsia healthcare resource use group (HRG) was used. When this was not possible, costs across HRGs were combined by weighting for frequency. Given the importance of length of stay for this analysis, all costs were calculated based on bed days or the minimum total cost for that HRG, whichever was greater. All costs are in 2018/2019 British Pounds. Costs are from an English NHS perspective.
QALYs were calculated as the area under the curve[15] using the EQ-5D-5L and the crosswalk to the EQ-5D-3L tariff [16] in line with National Institute for Health and Care Excellence (NICE) guidance to allow for comparability across disease areas [17]. Women that died before they reached a specific follow-up point were included as 0 for each follow-up point after they died, assuming a linear decline from their last complete questionnaire until death. The EQ-5D does not have a proxy reported version for children under 2 years of age and when the trial was designed there was no preference-based measure of health-related quality of life for children under 2 years of age. As we currently do not have sufficient information on infant health to base the calculation of a utility tariff, we assumed infants were in full health (utility of 1.0 at X, where X is any follow-up time point) for each infant. A utility tariff of 0 was applied for any infants that died based on the month of death. For example, if an infant had a date of death 3 months after birth, their total QALYs would be 1 × \(\frac{3}{12}\) = 0.25. Infant death was included alongside maternal QALYs as a decrement (maternal QALYs minus infant QALYs lost due to death) to obtain total QALYs. A secondary analysis was conducted where a disutility of 0.00753 was applied per infant inpatient bed day at 6 and 24 months [18].
Women also completed the Short Form 12 version 2 (SF-12v2®) Health Survey SF-12 at 6 months and 2 years post-delivery, which was converted to QALYs using the Short Form 6 dimension (SF-6D) algorithm [19]. This was included in a secondary analysis.
The mean differences in costs and QALYs, 95% CIs and p values were calculated using regression analysis adjusting for the minimisation factors of centre, singleton/twin pregnancy, severity of hypertension in 48 h prior to enrolment, parity, previous caesarean section and gestational age at randomisation. 95% CIs for adjusted differences were calculated using bootstrap with bias corrected and accelerated (BCA) CIs and 1000 repeated samples with replacement.
Costs and QALYs after 12 months were discounted at a rate of 3.5% [17].
2.4.1 Missing Data
For missing health and social care resource use items, it was assumed that if the questionnaire was returned or started online but the item was missing, they did not use that item, and hence, it was imputed as 0. Total 2-year costs were calculated for women and infants with data at both 6 months and 2 years.
For the EQ-5D-5L and SF-6D, if there were missing responses to any item required for calculating a utility tariff, the utility tariff for that individual was missing for that time point. Complete-case QALYs were calculated for individuals with utility tariffs at randomisation, 6 months and 2 years for the EQ-5D-5L and at 6 months and 2 years for the SF-6D.
The Statistical Analysis Plan for the primary long-term infant outcome, PARCA-R, was prespecified as a complete-case analysis. Given that missing data for the health economic evaluation is likely to follow the same pattern as for the primary long-term infant outcome, to align the two analyses, the primary analysis for the health economic analysis was also a complete-case analysis, testing for the assumption that data is missing at random. Published guidance on handling missing data in randomised controlled trials [20] and specifically in cost-effectiveness analysis alongside a randomised controlled trial [21] was used as guiding principles and to design sensitivity analyses of the results. Total costs and QALYs were adjusted for predictors of missingness in the primary analysis, in addition to minimisation factors and baseline utilities in the case of QALYs.
A sensitivity analysis was conducted where missing data was imputed using multiple imputation using chained equations (MICE).
2.4.2 ICER, CEAC and CEP
For the incremental cost-effectiveness ratio (ICER), seemingly unrelated regression (SUR Stata command SUREG) was used to account for the correlation between costs and outcomes to calculate the incremental mean cost per QALY gained of planned delivery compared to expectant management. The results were adjusted for minimisation factors (as specified above) and predictors of missingness. 95% CIs were calculated using bootstrap with BCA CIs and 5000 draws. The bootstrapped data were also used to calculate the cost-effectiveness plane (CEP) and cost-effectiveness acceptability curve (CEAC), the probability that planned initiation is cost-effective compared to usual care (expectant management) for a range of cost-effectiveness thresholds.
Sensitivity and secondary analyses are specified in the ESM. All analyses were conducted in STATA version 16.
3 Results
3.1 Trial Summary
Between 29 September 2014 and 10 December 2018, 901 women were enrolled to the trial, with 450 allocated to planned delivery and 451 allocated to expectant management (Fig. 1). Hospital clinical records for calculating short-term maternal and infant resource use and costs were available for 448 women (471 infants) allocated to planned delivery (as two of the allocated women withdrew consent) and 451 women (475 infants) allocated to expectant management. Baseline characteristics for the 899 women with complete short-term healthcare resource use data are reported in Table 2. At 24 months of follow-up, 355 women had complete QALYs calculated using the EQ-5D-5L: 186 (41%) allocated to planned delivery and 169 (38%) allocated to expectant management, and 400 women and their infants had complete resource use: 206 (46%) allocated to planned delivery and 194 (43%) allocated to expectant management. Women with complete data were more likely to be white, be older, have a lower deprivation of index score, and be less likely to smoke at baseline assessment compared to women who had missing data (see the ESM Table SM2). This was the same for both costs and QALYs.
3.2 Resource Use and Costs
Descriptive statistics for 24-month resource use are reported in the ESM, Tables SM2 and SM3. Neonatal resource use was a component of the perinatal co-primary outcome, so has been reported elsewhere, with an increase in the proportion admitted to an NNU for planned delivery (196/471 [42%] vs 159/475 [33%], odds ratio 1.26, 95% CI 1.08–1.47), but no significant difference in overall neonatal length of stay between groups [22]. Women in the planned delivery group spent significantly fewer nights on perinatal inpatient wards (adjusted difference − 3.52, 95% CI − 4.82 to − 2.22) and had significantly fewer obstetric outpatient appointments (adjusted difference − 1.78, 95% CI − 2.17 to − 1.49). There was evidence of a significant difference in short-term costs (updated for 2018/2019 costs) with planned delivery, on average, costing significantly less than expectant management (mean difference − £1988, 95% CI − 2887 to − 1089), the majority of which is accounted for in maternal care (mean difference − £2138, 95% CI − 2688 to − 1589), particularly antenatal inpatient stays (see Table 3). There were no significant differences in 6- or 24-month inpatient nights for infants (adjusted difference 0.02, 95% CI − 2.36 to 2.41; 2.47, 95% CI − 3.89 to 8.82, respectively) or costs for women or infants, but a significant difference when short-term and 24-month costs were combined (mean difference − £2280, 95% CI − 4437 to − 246).
3.3 QALYs
Descriptive statistics for EQ-5D-5L and the crosswalk algorithm are reported in Table 4. There were two infant deaths in the trial which were added to maternal QALYs as a decrement. There were no significant differences between the two groups in maternal QALYs (see Table 4). QALYs calculated using the SF-6D are reported in Table SM10 as part of secondary analysis 2.
3.4 ICER, CEAC and CEP
Using SUR to account for the correlation between costs and QALYs, and adjusting for minimisation factors and predictors of missingness, the mean discounted incremental cost per woman over 24 months for planned delivery compared to expectant management is − £2711 (BCA 95% CI − 4840 to − 637), with a mean incremental difference in QALYs (discounted) of 0.019 (BCA 95% CI − 0.039 to 0.063). Planned delivery dominates expectant management in that it costs less (significant difference) for more QALYs (non-significant difference). The CEP is reported in Fig. 2. There is a 99% probability that planned delivery is cost-effective compared to expectant management for all cost-effectiveness thresholds below £37,000 (see the ESM, Figure SM1), with £37,000 being the point at which the probability decreases below 99%.
The results remain consistent for all sensitivity and secondary analyses (see the ESM).
4 Discussion
4.1 Main Findings
There is a high probability that planned delivery is cost-effective compared to expectant management in pregnant women with a diagnosis of pre-eclampsia between 34+0 and 36+6 weeks’ gestation. This is predominately driven by cost-savings in antenatal maternal care. This is alongside clear maternal benefit with planned delivery (compared to expectant management), together with no short-term neonatal morbidity (although there is an increased proportion of NNU admissions). However, these increased admissions were not associated with greater costs, and there was no significant difference in neonatal costs, either on a short-term or 24-month time horizon.
Other studies that have evaluated placental growth factor testing as a diagnostic adjunct in the management of suspected pre-eclampsia have found that a majority of the cost in care comes from frequent maternal and foetal monitoring to detect adverse events [4]. In this trial, it is likely that the cost difference is due to planned delivery reducing the time to delivery (by around 5 days) and, hence, the duration during which costs associated with antenatal monitoring and adverse maternal outcomes can occur. This is alongside no significant difference in neonatal care costs.
4.2 Strengths and Limitations
The PHOENIX trial is the largest, randomised controlled trial in the literature to date evaluating planned delivery in late pre-term pre-eclampsia. We were able to collect complete resource use data for the majority of women and their infants from randomisation until postnatal discharge, the analysis of which shows significant cost savings associated with planned delivery compared to expectant management. We also have a relatively long follow-up of women and their infants to allow us to evaluate the 24-month impact of planned delivery at 34+0 and 36+6 weeks’ gestation, to ascertain whether planned delivery (increasing preterm delivery) or expectant management (increasing foetal growth restriction and need for emergency delivery) increases the risks of poor health outcomes and, hence, increased costs. There was no difference in costs for infants of mothers allocated to planned delivery compared to expectant management. There was no significant difference in infant costs at 24 months, although the degree of loss to follow-up limited certainty in this finding. This retention to follow-up is not uncommon for similar maternity trials with long-term follow-up of women and their infants, with follow-up rates varying from 14 to 61% [23,24,25]. The loss to follow-up though does reduce the strength of these findings and increases the risk of bias in the results. This is particularly true if the reasons for not responding were not by chance, for example, if there were more infants with severe illness in the non-responder group. However, in our study population, short-term infant outcomes between responders and non-responders at 2-year follow-up were similar with regards to NNU admission, birth of a small-for-gestational age (< 10th centile) infant and short-term morbidity such as the need for respiratory support (shown in the ESM and in linked paper). Given that we had a higher proportion of women and infants with complete data in the planned delivery group, this further reduces the chance that any imbalance would have a significant impact on the results. It is still of some concern that there is a higher proportion of missing data for women from more disadvantaged areas, even if this is adjusted for in the analysis.
Future trials should consider following up infants using routine data to improve retention rates, although our own experience is that it comes with substantial additional cost and complexity given that women and infants are reported separately in routine data.
A complete-case analysis was chosen prior to data analysis as our primary analysis. As discussed elsewhere [20, 21], stepping through from least to most complex analysis allows an evaluation of the impact of difference models on the results. The sensitivity analyses suggest that the results and interpretation of the analysis are robust to more complex models.
There remain questions regarding the suitability of the EQ-5D in maternity trials as it is unlikely to reflect maternal preferences for care. Preference based measures of health-related quality of life for infants under 12 months are also a challenging area of research, with measures that could be used to calculate QALYs in infants only recently published [26, 27] and, hence, could not be incorporated into this study. Other clinical outcomes used in the PHOENIX trial, such as the fullPIERS [28] for morbidity in pre-eclampsia and the PARCA-R for neurodevelopment at 2 years of age [29], may represent a fuller picture of the impact on clinical outcomes. These results are reported in the main clinical papers [8, 30], with no evidence of a clinically significant detriment in the planned delivery group at 2 years on the PARCA-R.
4.3 Interpretation
There are a limited number of economic evaluations of planned delivery versus expectant management in women with pre-eclampsia. The largest and most notable, the HYPITAT-II trial, found a non-significant higher cost for planned delivery compared to expectant management of €682 (95% CI − 618 to 2126) [31]. This was potentially driven by high NNU admissions (alongside low steroid use) in the planned delivery group. Although we also saw a high level of NNU admissions in the planned delivery group in the PHOENIX trial as the infants were delivered at less than 37 weeks, these infant admissions had a shorter length of stay, which meant that there was no significant difference in costs for the two groups. Steroid use was markedly higher in the PHOENIX trial (60%) compared to the HYPITAT-II trial (8%), likely influencing length of stay and neonatal morbidity in the trials.
Although 24 months is a longer follow-up than many pregnancy trials, it is possible that additional costs may occur beyond this time horizon, and a longer time horizon may be more appropriate. Although there is evidence for very pre-term birth (< 32 weeks’ gestation) health-related quality of life consequences into adolescence [32], there is less evidence for moderate to late pre-term birth. A recent systematic review found limited evidence available for the economic costs of pre-term birth beyond 24 months [33]. Studies that have looked beyond the 24-month time horizon, though, find that the hospitalisation costs associated with delivery still account for the majority of the costs, with reductions in the cost difference between pre-term and term births over time [33, 34]. Although studies have found an economic impact of late pre-term birth [35], these studies use general population > 37 weeks as controls, which do not take into account the potential impact of higher rates of foetal growth restriction in the expectant management group, an outcome that is also likely to result in long-term economic consequences [33]. These studies also do not find any difference in costs between pre-term and term births beyond the neonatal hospitalisation [35]. The impact on women’s health of an earlier delivery beyond 24 months is also not captured, as it is possible that this could reduce the long-term risk to cardiovascular health, although further evidence is needed [36].
We did not ask about wider societal costs, such as impact on employment and out-of-pocket costs, which are potentially large for this population group [5, 33], to keep our resource use questionnaire as brief as possible given the challenges associated with following up women in maternity trials. However, given no evidence of a difference between planned delivery and expectant management for any of the outcomes at 24 months, it is unlikely that we would have seen any difference between the two groups on wider societal cost outcomes, which is in keeping with other evidence [35].
5 Conclusions
This analysis provides evidence to decision makers that planned delivery is likely to be cost-effective compared to expectant management for women with pre-eclampsia between 34+0 and 36+6 weeks’ gestation. These findings need to be considered in the wider context of patient preferences and individual circumstances within maternity care. In conjunction with results around clinical outcomes, the demonstration of cost-effectiveness points towards the conclusion that patient/clinician conversations highlighting the benefits and risks of planned delivery are likely to be valuable to the healthcare service.
References
Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018;51(6):743–50.
Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–33.
Stevens W, Shih T, Incerti D, Ton TGN, Lee HC, Peneva D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237-248 e16.
Duhig KE, Seed PT, Myers JE, Bahl R, Bambridge G, Barnfield S, et al. Placental growth factor testing for suspected pre-eclampsia: a cost-effectiveness analysis. BJOG. 2019;126(11):1390–8.
Hao J, Hassen D, Hao Q, Graham J, Paglia MJ, Brown J, et al. Maternal and infant health care costs related to preeclampsia. Obstet Gynecol. 2019;134(6):1227–33.
Fox A, McHugh S, Browne J, Kenny LC, Fitzgerald A, Khashan AS, et al. Estimating the cost of preeclampsia in the healthcare system: cross-sectional study using data from SCOPE Study (Screening for Pregnancy End Points). Hypertension. 2017;70(6):1243–9.
Organisation WH. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. 2011. http://apps.who.int/iris/bitstream/10665/44703/1/9789241548335_eng.pdf.
Chappell LC, Brocklehurst P, Green ME, Hunter R, Hardy P, Juszczak E, et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet (Lond, Engl). 2019;394(10204):1181–90.
National Institute for Health and Clinical Excellence. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy; 2010.
Chappell LC, Green M, Marlow N, Sandall J, Hunter R, Robson S, et al. Planned delivery or expectant management for late preterm pre-eclampsia: study protocol for a randomised controlled trial (PHOENIX trial). Trials. 2019;20:1.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
Beecham J, Knapp M. Costing psychiatric interventions. In: Thornicroft G, editor. Measuring Mental Health Needs: Gaskell; 2001. p. 200–24.
NHS England. National Cost Collection for the NHS; 2020.
Curtis LA, Burns A. Unit costs of health and social care 2020. PSSRU: University of Kent; 2020.
Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. Pharmacoeconomics. 2015;33(4):355–66.
van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. 2013.
Kwon J, Kim SW, Ungar WJ, Tsiplova K, Madan J, Petrou S. A systematic review and meta-analysis of childhood health utilities. Med Decis Mak. 2018;38(3):277–305.
Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–9.
White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;7(342):d40.
Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32(12):1157–70.
Chappell LC, Brocklehurst P, Green ME, Hunter R, Hardy P, Juszczak E, et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394(10204):1181–90.
Brocklehurst P, Field D, Greene K, Juszczak E, Keith R, Kenyon S, et al. Computerised interpretation of fetal heart rate during labour (INFANT): a randomised controlled trial. Lancet. 2017;389(10080):1719–29.
Zwertbroek EF, Franssen MTM, Broekhuijsen K, Langenveld J, Bremer H, Ganzevoort W, et al. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring in mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. 2019;221(2):154.e1-.e11.
Zwertbroek EF, Zwertbroek J, Broekhuijsen K, Franssen MTM, Ganzevoort W, Langenveld J, et al. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring in mild hypertensive disorders of pregnancy: 5-year outcomes of the HYPITAT II trial. Eur J Obstet Gynecol Reprod Biol. 2020;244:172–9.
Krabbe PFM, Jabrayilov R, Detzel P, Dainelli L, Vermeulen KM, van Asselt ADI. A two-step procedure to generate utilities for the Infant health-related Quality of life Instrument (IQI). PLoS One. 2020;15(4):e0230852.
Jabrayilov R, Vermeulen KM, Detzel P, Dainelli L, van Asselt ADI, Krabbe PFM. Valuing health status in the first year of life: the infant health-related quality of life instrument. Value Health. 2019;22(6):721–7.
von Dadelszen P, Payne B, Li J, Ansermino JM, Broughton Pipkin F, Cote AM, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377(9761):219–27.
Johnson S, Marlow N, Wolke D, Davidson L, Marston L, O’Hare A, et al. Validation of a parent report measure of cognitive development in very preterm infants. Dev Med Child Neurol. 2004;46(6):389–97.
Beardmore-Gray A, Greenland M, Linsell L, Juszczak E, Hardy P, Placzek A, et al. Two-year follow-up of infant and maternal outcomes after planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. BJOG. 2022.
van Baaren GJ, Broekhuijsen K, van Pampus MG, Ganzevoort W, Sikkema JM, Woiski MD, et al. An economic analysis of immediate delivery and expectant monitoring in women with hypertensive disorders of pregnancy, between 34 and 37 weeks of gestation (HYPITAT-II). BJOG. 2017;124(3):453–61.
Petrou S, Krabuanrat N, Khan K. Preference-based health-related quality of life outcomes associated with preterm birth: a systematic review and meta-analysis. Pharmacoeconomics. 2020;38(4):357–73.
Petrou S, Yiu HH, Kwon J. Economic consequences of preterm birth: a systematic review of the recent literature (2009–2017). Arch Dis Child. 2019;104(5):456–65.
Korvenranta E, Linna M, Rautava L, Andersson S, Gissler M, Hallman M, et al. Hospital costs and quality of life during 4 years after very preterm birth. Arch Pediatr Adolesc Med. 2010;164(7):657–63.
Khan KA, Petrou S, Dritsaki M, Johnson SJ, Manktelow B, Draper ES, et al. Economic costs associated with moderate and late preterm birth: a prospective population-based study. BJOG. 2015;122(11):1495–505.
Staff AC. Long-term cardiovascular health after stopping pre-eclampsia. Lancet. 2019;394(10204):1120–1.
Acknowledgements
We thank the independent Trial Steering Committee [chair, Jane Norman (University of Bristol); members, Simon Gates (University of Birmingham), Alison Leaf (University Hospital Southampton NHS Foundation Trust), Katie Lean (Oxford University Hospitals NHS Foundation Trust), Stavros Petrou (University of Oxford) and Jacqui Williams (lay member)] and the independent Data Monitoring Committee [chair, Diana Elbourne (London School of Hygiene & Tropical Medicine); members, Phillip Bennett (Imperial College London) and Jon Dorling (Dalhousie University, Halifax)]. We also thank all the participating women, site research midwives and doctors for their contribution to the trial.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This paper presents independent research commissioned by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project reference 12/25/03). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflict of interest
NM reports personal fees from Shire and Novartis, outside of the submitted work. All other authors report no conflict of interest.
Availability of data and material
The dataset will be available to appropriate academic parties on request from the Chief Investigator (LCC) in accordance with the data sharing policies of King's College London and the National Perinatal Epidemiology Unit Clinical Trials Unit, with input from the co-investigator group where applicable.
Ethics approval
The trial was approved by the South Central-Hampshire B Research Ethics Committee (no13/SC/0645).
Author contributions
LCC, RH, EJ, PH, NM and AS were involved in the study conception and in securing funding for the study. LCC and AS were co-chief investigators responsible for all aspects of the study. RH did the health economic analysis with input from MG and LL on the statistical component. LCC, EJ and NM provided advice on the analysis. AP made a substantial contribution to the running of the trial. RH, ABG and LCC wrote the article. All authors reviewed, contributed to, and approved the final version of the manuscript.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hunter, R., Beardmore-Gray, A., Greenland, M. et al. Cost-Utility Analysis of Planned Early Delivery or Expectant Management for Late Preterm Pre-eclampsia (PHOENIX). PharmacoEconomics Open 6, 723–733 (2022). https://doi.org/10.1007/s41669-022-00355-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00355-1