Abstract
Infiltration systems are increasingly used to reduce peak flows and mitigate the impacts of stormwater runoff. Despite the benefits of infiltration systems, there is a risk for associated pollutants, including heavy metals to be introduced to the underlying soil and groundwater. The subsequent movement of metals in the subsurface and their potential to contaminate water resources is uncertain and profoundly depends on the adsorptive behavior of the surrounding soil. We used two soil types, one natural (quartz–kaolinite–muscovite) and one synthetic (quartz and kaolinite only) with different clay mineralogy to test their potential adsorptive capacities through batch systems, with Zn(II) as a representative tracer. Nonlinear isotherms, Freundlich and Langmuir, provided good fits with the experimental sorption data. Kinetic data were well fitted by a pseudo-second-order model, indicating that cation exchange exists between the clay surfaces and Zn(II) in the liquid phase. We found that the natural soil adsorbed far more Zn(II) when compared to the synthetic soil which was attributed to the presence of the muscovite in the natural soil. Comparison of the observed adsorption capacity of the two soils with their predicted adsorption capacities showed that while the adsorption capacities of the single-sized clay minerals are widely reported, these values cannot be linearly extrapolated to estimate the adsorption capacity of a soil that might contain varied fractions of clay. The results suggest that the designers of infiltration systems should first undertake an analysis of clay mineralogy of the subsurface soil to better predict the fate of heavy metals within the surrounding soils.
Article Highlights
-
We investigate the impact of soil clay minerals on zinc ion immobilization.
-
Muscovite significantly contributes to zinc ion sorption.
-
The soil sorption capacity cannot be estimated from its individual mineral sorption capacities.
-
Sorption of zinc ion in the soil is chemically rather than physically modulated.








Similar content being viewed by others
Notes
Inductively coupled plasma optical emission spectroscopy.
References
Al-Ameri M, Hatt B, Le Coustumer S, Fletcher T, Payne E, Deletic A (2018) Accumulation of heavy metals in stormwaterbioretention media: a field study of temporal and spatial variation. J Hydrol 567:721–731
Al-Degs YS, El-Barghouthi MI, Issa AA, Khraisheh MA, Walker GM (2006) Sorption of Zn(II), Pb(II), and Co(II) using natural sorbents: equilibrium and kinetic studies. Water Res 40(14):2645–2658
Alloway BJ (2013) Sources of heavy metals and metalloids in soils. Heavy metals in soils. Springer, pp 11–50
Altin O, Ozbelge OH, Dogu T (1999) Effect of pH, flow rate and concentration on the sorption of Pb and Cd on montmorillonite: I. Experimental. J ChemTechnolBiotechnol 74(12):1131–1138
Arias F, Sen TK (2009) Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay mineral: a kinetic and equilibrium study. Colloids Surf A 348(1–3):100–108
ATSDR (2005) Toxicological profile for zinc. US Department of Health and Human Services, Public Health Services (Agency for Toxic Substances and Disease Registry), Atlanta, GA
Barraud S, Gautier A, Bardin J-P, Riou V (1999) The impact of intentional stormwater infiltration on soil and groundwater. Water SciTechnol 39(2):185–192
Barton C (2002) Clay minerals. In: Rattan Lal, comp. (ed) Encyclopedia of soil science. Marcel Dekker, New York, pp 187–192
Bereket G, Arog AZ, Özel MZ (1997) Removal of Pb(II), Cd(II), Cu(II), and Zn(II) from aqueous solutions by adsorption on bentonite. J Colloïd Interface Sci 187(2):338–343
Bhattacharyya KG, Gupta SS (2008) Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium. Appl Clay Sci 41(1–2):1–9
Bonneau J, Fletcher TD, Costelloe JF, Poelsma PJ, James RB, Burns MJ (2018) Where does infiltrated stormwater go? Interactions with vegetation and subsurface anthropogenic features. J Hydrol 567:121–132
Brigatti M, Galan E, Theng B (2013) Structure and mineralogy of clay minerals. Dev Clay Sci 5:21–81
Burham N, Sayed M (2016) Adsorption behavior of Cd2+ and Zn2+ onto natural Egyptian bentonitic clay. Minerals 6(4):129
Chai W, Huang Y, Su S, Han G, Liu J, Cao Y (2017) Adsorption behavior of Zn(II) onto natural minerals in wastewater. A comparative study of bentonite and kaolinite. Physicochem Probl Miner Process 53
Charles T, Tapiwa H (2017) Heavy metal contamination of ground water from an unlined landfill in Bulawayo, Zimbabwe. J Health Pollut 8(15):18–27. https://doi.org/10.5696/2156-9614-8.15.18
Clark SE, Pitt R (2007) Influencing factors and a proposed evaluation methodology for predicting groundwater contamination potential from stormwater infiltration activities. Water Environ Res 79(1):29–36
Crini G, Peindy HN, Gimbert F, Robert C (2007) Removal of CI Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep PurifTechnol 53(1):97–110
Diagboya PN, Olu-Owolabi BI, Adebowale KO (2015) Effects of time, soil organic matter, and iron oxides on the relative retention and redistribution of lead, cadmium, and copper on soils. Environ SciPollut Res 22(13):10331–10339
Fischer D, Charles EG, Baehr AL (2003) Effects of stormwater infiltration on quality of groundwater beneath retention and detention basins. J Environ Eng 129(5):464–471
Fletcher TD, Shuster W, Hunt WF, Ashley R, Butler D, Arthur S, Bertrand-Krajewski J-L et al (2015) SUDS, LID, BMPs, WSUD and more—the evolution and application of terminology surrounding urban drainage. Urban Water J 12(7):525–542
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. ChemEng J 156(1):2–10
Galitskaya IV, Mohan KR, Krishna AK, Batrak GI, Eremina ON, Putilina VS, Yuganova TI (2017) Assessment of soil and groundwater contamination by heavy metals and metalloids in russian and indian megacities. Procedia Earth Planet Sci 17:674–677. https://doi.org/10.1016/j.proeps.2016.12.180
Grim RE (1968) Clay mineralogy: international series in the earth and planetary sciences. McGraw-Hill, New York
Gupta SS, Bhattacharyya KG (2008) Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J Environ Manag 87(1):46–58
Hamel P, Daly E, Fletcher TD (2013) Source-control stormwater management for mitigating the impacts of urbanisation on baseflow: a review. J Hydrol 485:201–211
Ho Y-S, McKay G (1998) Sorption of dye from aqueous solution by peat. ChemEng J 70(2):115–124
Ho Y-S, McKay G (2002) Application of kinetic models to the sorption of copper(II) on to peat. AdsorptSciTechnol 20(8):797–815
Ijagbemi CO, Baek M-H, Kim D-S (2009) Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater 166(1):538–546
Inglezakis VJ, Loizidou MD, Grigoropoulou HP (2003) Ion exchange of Pb(2+), Cu(2+), Fe(3+), and Cr(3+) on natural clinoptilolite: selectivity determination and influence of acidity on metal uptake. J Colloid Interface Sci 261(1):49–54
Jain M, Garg V, Kadirvelu K (2009) Chromium (VI) removal from aqueous system using Helianthus annuus (sunflower) stem waste. J Hazard Mater 162(1):365–372
Jiang M-Q, Jin X-Y, Lu X-Q, Chen Z-L (2010) Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252(1–3):33–39
Kyzioł-Komosińska J, Rosik-Dulewska C, Dzieniszewska A, Pająk M, Krzyżewska I (2014) Removal of Cr(III) ions from water and wastewater by sorption onto peats and clays occurring in an overburden of lignite beds in central Poland. Environ ProtEng. https://doi.org/10.37190/epe140101
Lagergreen S (1898) About the kinetic of so called adsorption of soluble substances. K. Sven. Vetenskapsakad. Handl.
Limousin G, Gaudet J-P, Charlet L, Szenknect S, Barthes V, Krimissa M (2007) Sorption isotherms: a review on physical bases, modeling and measurement. ApplGeochem 22(2):249–275
Mason Y, Ammann AA, Ulrich A, Sigg L (1999) Behavior of heavy metals, nutrients, and major components during roof runoff infiltration. Environ SciTechnol 33(10):1588–1597
Miranda-Trevino JC, Coles CA (2003) Kaolinite properties, structure and influence of metal retention on pH. Appl Clay Sci 23(1):133–139
Ogiyama S, Sakamoto K, Suzuki H, Ushio S, Anzai T, Inubushi K (2005) Accumulation of zinc and copper in an arable field after animal manure application. Soil Sci Plant Nutr (Japan) 51(6):801–808
Olu-Owolabi BI, Diagboya PN, Unuabonah EI, Alabi AH, Düring R-A, Adebowale KO (2018) Fractal-like concepts for evaluation of toxic metals adsorption efficiency of feldspar-biomass composites. J Clean Prod 171:884–891
Pitt R, Clark S, Field R (1999) Groundwater contamination potential from stormwater infiltration practices. Urban water 1(3):217–236
Purushotham D, Linga D, Sagar N, Mishra S, Naga Vinod G, Venkatesham K, Saikrishna K (2017) Groundwater contamination in parts of Nalgonda district, Telangana, India as revealed by trace elemental studies. J GeolSoc India 90(4):447–458. https://doi.org/10.1007/s12594-017-0738-0
Rayment GE, Lyons DJ (2011) Soil chemical methods: Australasia. Australian soil and land survey handbooks, vol 3. CSIRO Publishing, p 495
Roy JW, Bickerton G (2012) Toxic groundwater contaminants: an overlooked contributor to urban stream syndrome? Environ SciTechnol 46(2):729–736
Sánchez-Martín M, Dorado M, Del Hoyo C, Rodríguez-Cruz M (2008) Influence of clay mineral structure and surfactant nature on the adsorption capacity of surfactants by clays. J Hazard Mater 150(1):115–123
Shukla A, Zhang Y-H, Dubey P, Margrave J, Shukla SS (2002) The role of sawdust in the removal of unwanted materials from water. J Hazard Mater 95(1–2):137–152
Srilert C, Pokatte W, Wattasit S (2013) Long-term effects of fertilizer applications on heavy metals contaminations in groundwater and health risk assessment in the agricultural area, Ubon Ratchthani Province. p 16
Taty-Costodes VC, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater 105(1–3):121–142
Treybal RE (1980) Mass transfer operations. New York, 466
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. ChemEng J 308:438–462
Unuabonah E, Olu-Owolabi B, Adebowale K (2016) Competitive adsorption of metal ions onto goethite–humic acid-modified kaolinite clay. Int J Environ SciTechnol 13(4):1043–1054
Velde B (1995) Composition and mineralogy of clay minerals. Origin and mineralogy of clays. Springer, pp 8–42
Veli S, Alyüz B (2007) Adsorption of copper and zinc from aqueous solutions by using natural clay. J Hazard Mater 149(1):226–233
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP II (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am BentholSoc 24(3):706–723
Warren BE (1990) X-ray diffraction. Courier Corporation
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20(2):228–238
Weber WJ, McGinley PM, Katz LE (1992) A distributed reactivity model for sorption by soils and sediments. 1. Conceptual basis and equilibrium assessments. Environ SciTechnol 26(10):1955–1962
Weiss PT, LeFevre G, Gulliver JS (2008) Contamination of soil and groundwater due to stormwater infiltration practices. A literature review. Minnesota Pollution Control Agency, St. Paul
Xu L, Tian J, Wu H, Fang S, Lu Z, Ma C, Hu Y et al (2018) Anisotropic surface chemistry properties and adsorption behavior of silicate mineral crystals. AdvColl Interface Sci 256:340–351
Acknowledgements
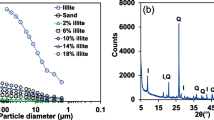
We thank the Materials Characterization and Fabrication Platform (MCFP) at the University of Melbourne for the XRD analysis and acknowledge the financial support of ARC DP170102870 for field and laboratory works. We also express our thanks to Mr. Murray Joseph for providing us silica sand 50N, silica flour 100G and kaolin K10 produced in Sibelco Australia Ltd. Co. Behroozi was supported by the Melbourne International Fee Remission Scholarship (MIFRS) and Melbourne International Research Scholarship (MIRS) from the University of Melbourne. Fletcher was supported by an ARC Future Fellowship (FT100100144) during part of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Behroozi, A., Arora, M., Fletcher, T.D. et al. Understanding the Impact of Soil Clay Mineralogy on the Adsorption Behavior of Zinc. Int J Environ Res 15, 559–569 (2021). https://doi.org/10.1007/s41742-021-00334-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-021-00334-0