Key summary points
To summarize current evidence on osteosarcopenia.
AbstractSection FindingsKnowledge on osteosarcopenia as a geriatric syndrome is growing. This will facilitate the development of robust biomarkers and new treatments with dual effect on muscle and bone.
AbstractSection MessageWhen assessing older persons for osteoporosis, concomitant assessment for sarcopenia (and vice versa) should be included as an essential part of the comprehensive geriatric assessment.
Abstract
Purpose
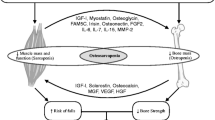
Osteosarcopenia is a geriatric syndrome characterized by declines in bone density and microarchitecture and muscle mass and strength, which has gained clinical interest due to its association with falls and fragility fractures.
Methods
This review discusses the epidemiology of osteosarcopenia including clinical assessment, the pathophysiological aspects leading to the loss of muscle and bone mass, and efficacious therapeutic strategies to combat this syndrome.
Results
The etiology of osteosarcopenia is thought to include genetic and environmental factors which interact with muscle and bone at the cellular level, reinforcing that these tissues are interconnected not only by mechanical aspects, but also by humoral factors. Osteosarcopenia is identified by low muscle and bone mass and impaired strength of these tissues via imaging and physical performance measures.
Conclusion
The diagnosis of osteosarcopenia is of clinical importance since early interventions, particularly resistance exercise, and adequate intake of protein, vitamin D and calcium, may delay the onset of individual components (osteopenia/sarcopenia) of osteosarcopenia.

Sourced from Kirk et al. [35]

Similar content being viewed by others
References
Nielsen BR, Abdulla J, Andersen HE, Schwarz P, Suetta C (2018) Sarcopenia and osteoporosis in older people: a systematic review and meta-analysis. Eur Geriatr Med 9:419–434
Sozen T, Ozisik L, Basaran NC (2017) An overview and management of osteoporosis. Eur J Rheumatol. 4:46–56
Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C (2015) Osteoporosis and sarcopenia in older age. Bone 80:126–130
Hirschfeld HP, Kinsella R, Duque G (2017) Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 28:2781–2790
Binkley N, Buehring B (2009) Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitom. 12:413–416
Crepaldi G, Maggi S (2005) Sarcopenia and osteoporosis: a hazardous duet. J Endocrinol Invest 28:66–68
Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB (2010) Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 21:543–559
Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L et al (2008) A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23:205–214
Curtis E, Litwic A, Cooper C, Dennison E (2015) Determinants of muscle and bone aging. J Cell Physiol 230:2618–2625
Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V (2015) Muscle and bone, two interconnected tissues. Ageing Res Rev. 21:55–70
Bonewald L (2019) Use it or lose it to age: a review of bone and muscle communication. Bone 120:212–218
Kobayashi K, Imagama S, Ando K et al (2020) Epidemiology and effect on physical function of osteosarcopenia in community-dwelling elderly people in Japan. Mod Rheumatol. 30(3):592–597
Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R, Fi ATig (2016) Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 28:895–899
Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Muir SW et al (2015) Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc. 16:290–295
Poggiogalle E, Cherry KE, Su LJ, Kim S, Myers L, Welsh DA et al (2019) Body Composition, IGF1 Status, and Physical Functionality in Nonagenarians: implications for Osteosarcopenia. J Am Med Dir Assoc. 20:70–75
Yu R, Leung J, Woo J (2014) Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc. 15:551–558
Yoshimura N, Muraki S, Oka H, Iidaka T, Kodama R, Horii C et al (2018) Do sarcopenia and/or osteoporosis increase the risk of frailty? A 4-year observation of the second and third ROAD study surveys. Osteoporos Int 29:2181–2190
Kull M, Kallikorm R, Lember M (2012) Impact of a new sarco-osteopenia definition on health-related quality of life in a population-based cohort in Northern Europe. J Clin Densitom. 15:32–38
Yoo JI, Kim H, Ha YC, Kwon HB, Koo KH (2018) Osteosarcopenia in patients with hip fracture is related with high mortality. J Korean Med Sci. https://doi.org/10.3346/jkms.2018.33.e27
Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA (2005) Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Mol Aspects Med 26:181–201
Laurent MR, Dubois V, Claessens F, Verschueren SM, Vanderschueren D, Gielen E et al (2016) Muscle-bone interactions: from experimental models to the clinic? A critical update. Mol Cell Endocrinol. 432:14–36
Karasik D, Kiel DP (2010) Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone 46:1226–1237
De Rui M, Inelmen EM, Pigozzo S, Trevisan C, Manzato E, Sergi G (2019) Dietary strategies for mitigating osteosarcopenia in older adults: a narrative review. Aging Clin Exp Res 31:897–903
Wintermeyer E, Ihle C, Ehnert S, Stockle U, Ochs G, de Zwart P et al (2016) Crucial role of vitamin D in the musculoskeletal system. Nutrients. https://doi.org/10.3390/nu8060319
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism. J Cell Biochem 116:687–695
Laurent MR, Dedeyne L, Dupont J, Mellaerts B, Dejaeger M, Gielen E (2019) Age-related bone loss and sarcopenia in men. Maturitas. 122:51–56
Sirola J, Kroger H (2011) Similarities in acquired factors related to postmenopausal osteoporosis and sarcopenia. J Osteoporos. 2011:536735. https://doi.org/10.4061/2011/536735
Landi F, Onder G, Bernabei R (2013) Sarcopenia and diabetes: two sides of the same coin. J Am Med Dir Assoc. 14:540–541
Picke AK, Campbell G, Napoli N, Hofbauer LC, Rauner M (2019) Update on the impact of type 2 diabetes mellitus on bone metabolism and material properties. Endocr Connect. 8:R55–R70
Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A et al (2014) Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle. 5:183–192
Maurel DB, Jahn K, Lara-Castillo N (2017) Muscle-bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines. https://doi.org/10.3390/biomedicines5040062
Elkina Y, von Haehling S, Anker SD, Springer J (2011) The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2:143–151
Kaji H (2016) Effects of myokines on bone. Bonekey Rep. https://doi.org/10.1038/bonekey.2016.48
Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H, Cardozo C et al (2017) Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J Biol Chem 292:11021–11033
Kirk B, Al Saedi A, Duque G (2019) Osteosarcopenia: A case of geroscience. Aging Med (Milton). 2(3):147–156. https://doi.org/10.1002/agm2.12080
Demontiero O, Boersma D, Suriyaarachchi P et al (2014) clinical outcomes of impaired muscle and bone interactions. Clinic Rev Bone Miner Metab 12:86–92
Fatima M, Brennan-Olsen SL, Duque G (2019) Therapeutic approaches to osteosarcopenia: insights for the clinician. Ther Adv Musculoskelet Dis. https://doi.org/10.1177/1759720X19867009
Cruz-Jentoft AJ, Sayer AA (2019) Sarcopenia. The Lancet. 393:2636–2646
Zanker J, Duque G (2019) Osteoporosis in older persons: old and new players. J Am Geriatr Soc 67:831–840
Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for C, Economic Aspects of O et al (2019) Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif Tissue Int. 104:235–238
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE (2016) SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 7:28–36
Edwards MH, Gregson CL, Patel HP, Jameson KA, Harvey NC, Sayer AA et al (2013) Muscle size, strength, and physical performance and their associations with bone structure in the Hertfordshire Cohort Study. J Bone Miner Res 28:2295–2304
La Tegola L, Mattera M, Cornacchia S, Cheng X, Guglielmi G (2018) Diagnostic imaging of two related chronic diseases: sarcopenia and Osteoporosis. J Frailty Sarcopenia Falls. 03:139–147
Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM (2019) D3 -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 10:14–21
Chalhoub D, Cawthon PM, Ensrud KE, Stefanick ML, Kado DM, Boudreau R et al (2015) Risk of nonspine fractures in older adults with sarcopenia, low bone mass, or both. J Am Geriatr Soc 63:1733–1740
Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM et al (2019) Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci 74:844–852
Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM et al (2019) Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 10:485–500
Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K et al (2019) Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012424.pub2
Daly RM, Gianoudis J, Kersh ME, Bailey CA, Ebeling PR, Krug R et al (2020) Effects of a 12-Month supervised, community-based, multimodal exercise program followed by a 6-month research-to-practice transition on bone mineral density, trabecular microarchitecture, and physical function in older adults: a Randomized Controlled Trial. J Bone Miner Res 35:419–429
Kirk B, Mooney K, Amirabdollahian F, Khaiyat O (2019) Exercise and dietary-protein as a countermeasure to skeletal muscle weakness: Liverpool Hope University-Sarcopenia Aging Trial (LHU-SAT). Front Physiol. https://doi.org/10.3389/fphys.2019.00445
Lee N, Choi CJ (2019) Smoking and diabetes as predictive factors of accelerated loss of muscle mass in middle-aged and older women: a six-year retrospective cohort study. J Womens Health (Larchmt). 28:1391–1398
Maurel DB, Boisseau N, Benhamou CL, Jaffre C (2012) Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int 23:1–16
Hong AR, Kim SW (2018) Effects of Resistance Exercise on Bone Health. Endocrinol Metab (Seoul). 33:435–444
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C et al (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000333.pub2
Vikberg S, Sorlen N, Branden L, Johansson J, Nordstrom A, Hult A et al (2019) Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: a randomized controlled trial. J Am Med Dir Assoc. 20:28–34
Vlietstra L, Hendrickx W, Waters DL (2018) Exercise interventions in healthy older adults with sarcopenia: a systematic review and meta-analysis. Australas J Ageing. 37:169–183
Liu CJ, Latham NK (2009) Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002759.pub2
Finnegan S, Seers K, Bruce J (2019) Long-term follow-up of exercise interventions aimed at preventing falls in older people living in the community: a systematic review and meta-analysis. Physiotherapy. 105:187–199
Darling AL, Manders RJF, Sahni S, Zhu K, Hewitt CE, Prince RL et al (2019) Dietary protein and bone health across the life-course: an updated systematic review and meta-analysis over 40 years. Osteoporos Int 30:741–761
Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE et al (2013) Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 14:542–559
Breen L, Phillips SM (2011) Skeletal muscle protein metabolism in the elderly: interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond). https://doi.org/10.1186/1743-7075-8-68
Guescini M, Tiano L, Genova ML, Polidori E, Silvestri S, Orlando P et al (2017) The Combination of physical exercise with muscle-directed antioxidants to counteract sarcopenia: a biomedical rationale for pleiotropic treatment with creatine and coenzyme Q10. Oxid Med Cell Longev. https://doi.org/10.1155/2017/7083049
Candow DG, Forbes SC, Chilibeck PD, Cornish SM, Antonio J, Kreider RB (2019) Effectiveness of creatine supplementation on aging muscle and bone: focus on falls prevention and inflammation. J Clin Med. https://doi.org/10.3390/jcm8040488
Lewis JR, Sim M, Daly RM (2019) The vitamin D and calcium controversy: an update. Curr Opin Rheumatol 31:91–97
Chiodini I, Bolland MJ (2018) Calcium supplementation in osteoporosis: useful or harmful? Eur J Endocrinol 178:D13–D25
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Chapuy MC, Arlot ME, Duboeuf F et al (1992) Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 327:1637–1642
Jackson RD, LaCroix AZ, Gass M et al (2006) Calcium plus vitamin D supplementation and the risk of fractures [published correction appears in. N Engl J Med 354:669–683
Yao P, Bennett D, Mafham M, Lin X, Chen Z, Armitage J et al (2019) Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open. https://doi.org/10.1001/jamanetworkopen.2019.17789
Grant AM, Avenell A, Campbell MK et al (2005) Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 365:1621–1628
Duque G, Daly RM, Sanders K, Kiel DP (2017) Vitamin D, bones and muscle: myth versus reality. Australas J Ageing. 36(Suppl 1):8–13
McClung MR (2017) Denosumab for the treatment of osteoporosis. Osteoporos Sarcopenia. 3:8–17
Bilezikian JP, Lin CJF, Brown JP, Wang AT, Yin X, Ebeling PR et al (2019) Long-term denosumab treatment restores cortical bone loss and reduces fracture risk at the forearm and humerus: analyses from the FREEDOM Extension cross-over group. Osteoporos Int 30:1855–1864
Dufresne SS, Dumont NA, Boulanger-Piette A, Fajardo VA, Gamu D, Kake-Guena SA et al (2016) Muscle RANK is a key regulator of Ca2 + storage, SERCA activity, and function of fast-twitch skeletal muscles. Am J Physiol Cell Physiol 310:C663–C672
Dufresne SS, Boulanger-Piette A, Bossé S, Frenette J (2016) Physiological role of receptor activator nuclear factor-kB (RANK) in denervation-induced muscle atrophy and dysfunction. Recept Clin Investig 3:e13231–e13236
Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S (2019) RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 129:3214–3223
De Spiegeleer A, Beckwee D, Bautmans I, Petrovic M, Sarcopenia Guidelines Development group of the Belgian Society of G, Geriatrics (2018) Geriatrics pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Drugs Aging. 35:719–734
Barake M, Arabi A, Nakhoul N, El-Hajj Fuleihan G, El Ghandour S, Klibanski A et al (2018) Effects of growth hormone therapy on bone density and fracture risk in age-related osteoporosis in the absence of growth hormone deficiency: a systematic review and meta-analysis. Endocrine 59:39–49
Sullivan DH, Carter WJ, Warr WR, Williams LH (1998) Side effects resulting from the use of growth hormone and insulin-like growth factor-I as combined therapy to frail elderly patients. J Gerontol A Biol Sci Med Sci 53:M183–M187
Laurent MR, Jardi F, Dubois V, Schollaert D, Khalil R, Gielen E et al (2016) Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Bone 93:33–42
Magnussen LV, Hvid LG, Hermann AP, Hougaard DM, Gram B, Caserotti P et al (2017) Testosterone therapy preserves muscle strength and power in aging men with type 2 diabetes-a randomized controlled trial. Andrology. 5:946–953
Narayanan R, Coss CC, Dalton JT (2018) Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol 465:134–142
Clark RV, Walker AC, Andrews S, Turnbull P, Wald JA, Magee MH (2017) Safety, pharmacokinetics and pharmacological effects of the selective androgen receptor modulator, GSK2881078, in healthy men and postmenopausal women. Br J Clin Pharmacol 83:2179–2194
Morimoto M, Amano Y, Oka M, Harada A, Fujita H, Hikichi Y et al (2017) Amelioration of sexual behavior and motor activity deficits in a castrated rodent model with a selective androgen receptor modulator SARM-2f. PLoS one. https://doi.org/10.1371/journal.pone.0189480
Long KK, O’Shea KM, Khairallah RJ, Howell K, Paushkin S, Chen KS et al (2019) Specific inhibition of myostatin activation is beneficial in mouse models of SMA therapy. Hum Mol Genet 28:1076–1089
Camporez JP, Petersen MC, Abudukadier A, Moreira GV, Jurczak MJ, Friedman G et al (2016) Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci USA. 113:2212–2217
Tang L, Gao X, Yang X, Zhang D, Zhang X, Du H et al (2016) Combination of weight-bearing training and anti-MSTN Polyclonal antibody improve bone quality in rats. Int J Sport Nutr Exerc Metab 26:516–524
Kirk B, Zanker J, Duque G (2020) Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle. https://doi.org/10.1002/jcsm.12567
Kanis JA, Hans D, Cooper C, Baim S, Bilezikian JP, Binkley N et al (2011) Interpretation and use of FRAX in clinical practice. Osteoporos Int 22:2395–2411
Sandhu SK, Nguyen ND, Center JR, Pocock NA, Eisman JA, Nguyen TV (2010) Prognosis of fracture: evaluation of predictive accuracy of the FRAX algorithm and Garvan nomogram. Osteoporos Int 21:863–871
Acknowledgements
The authors would like to thank the Australian Institute for Musculoskeletal Science (AIMSS) for providing support for this study.
Author information
Authors and Affiliations
Contributions
GFB and EAPS reviewed the literature, while GFB primarily wrote the paper overseen by BK and GD. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Ethical permission was not required for this review article.
Informed consent
Informed consent is not required for review articles.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fagundes Belchior, G., Kirk, B., Pereira da Silva, E.A. et al. Osteosarcopenia: beyond age-related muscle and bone loss. Eur Geriatr Med 11, 715–724 (2020). https://doi.org/10.1007/s41999-020-00355-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-020-00355-6