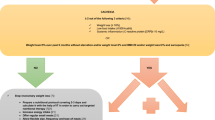
Key summary points
The aim of this retrospective cohort study was to investigate the impact of early postoperative factors on change in skeletal muscle mass 4 months after curative esophagectomy in older patients with esophageal cancer.
AbstractSection FindingsThe change (per 1%) in quadriceps muscle strength in the first month after surgery (standardized β = 0.190, p = 0.048) impacted the change (per 1%) in skeletal muscle mass 4 months after surgery, which was independent of age, sex, preoperative skeletal muscle mass, comorbidity, pathological stage, and neoadjuvant chemotherapy.
AbstractSection MessageWe believe that our findings will progress the development of novel comprehensive rehabilitation, including exercise and nutrition therapy after the perioperative phase in older patients with esophageal cancer.
Abstract
Background
Loss of skeletal muscle mass, measured by the skeletal muscle mass index (SMI), after esophagectomy negatively impacts prognosis. However, the information to develop novel supportive care options for preventing loss of skeletal muscle mass is limited. The purpose of this retrospective cohort study was to investigate the impact of early postoperative factors on change in SMI 4 months after curative esophagectomy in older patients with esophageal cancer.
Methods
This study included 113 subjects who underwent esophagectomy between 2015 and 2020. Preoperative and postoperative SMI (cm2/m2) were calculated from computed tomography images. The percentage change in SMI 4 months after surgery (SMI%) was calculated as follows: ([postoperative SMI – preoperative SMI] ÷ preoperative SMI) × 100. Potential factors affecting percentage change of SMI after surgery were analyzed by multiple regression.
Results
The mean SMI% was – 5.6%. The percentage change (per 1%) in quadriceps muscle strength in the first month after surgery (standardized β = 0.190, p = 0.048) impacted the SMI%, which was independent of age, sex, preoperative SMI, comorbidity, pathological stage, and neoadjuvant chemotherapy.
Conclusion
Quadriceps muscle weakness in the first month after esophagectomy impacted the SMI% in a dose-dependent relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 5-year survival rate of patients who undergo curative esophagectomy for esophageal cancer (EC) is 30–83% [1], which is worse than for other cancers. In addition, the number of older patients with EC has been growing, and older patients have poorer prognoses than younger patients [2, 3]. There is a global need for research into potential interventions to improve the prognosis and health of older patients with cancer.
In older patients with cancer, skeletal muscle mass is an important prognostic factor [4, 5]. It was recently reported that in older patients with EC, a change in skeletal muscle mass 4 months after surgery is an important factor for overall survival independent of preoperative skeletal muscle mass [6, 7]. Additionally, the previous study also showed that the association between postoperative change in skeletal muscle mass (per 1%) and overall survival in older patients with EC had a strong dose-dependent relationship [7]. Although preoperative factors, including pathological tumor stage and preoperative skeletal muscle mass, impacted postoperative changes in skeletal muscle mass in previous studies [6, 7], to our knowledge, no studies have investigated the impact of early postoperative factors on postoperative changes in skeletal muscle mass. Changes in skeletal muscle mass are associated with reversible factors, such as physical function and nutrition, and irreversible factors, such as disease and aging, in older adults [4, 8, 9]. If early postoperative physical function and nutrition affect changes in skeletal muscle mass 4 months after surgery in older patients with EC, appropriate supportive care and rehabilitation may prevent critical loss of skeletal muscle mass.
The present study aimed to investigate the impacts of early postoperative factors on changes in skeletal muscle mass 4 months after curative esophagectomy in older patients with EC.
Methods
Design and participants
This study was a retrospective cohort study in patients aged 65 years or older who had undergone curative esophagectomy and physical function examination for EC at the National Cancer Center East Hospital in Japan between September 2015 and December 2020. Esophagectomy with three-field lymph node dissection was performed via open surgery or minimally invasive surgery. Enteral feeding was administered through a feeding tube placed in the jejunum in all patients. Seven days after surgery, all patients underwent a contrast study to identify any anastomotic leakage. If there was no leakage, oral fluid intake was started immediately. In patients with clinical stage II and III (Union for International Cancer Control tumor–node–metastasis [UICC-TNM] classification, 7th edition) EC, neoadjuvant chemotherapy (NAC) with cisplatin and fluorouracil or docetaxel, cisplatin, and fluorouracil was administered according to individual patient tolerance. Perioperative rehabilitation was performed on all patients. Preoperative rehabilitation was performed as home-based interventions consisting of respiratory training, resistance training, and aerobic exercise. Postoperative rehabilitation was performed from the first postoperative day at an intensive care unit up until discharge for 20–40 min per day, which included early mobilization, respiratory training, resistance training, and aerobic exercise.
The exclusion criteria were as follows: R1–2 curative esophagectomy; untreated or undertreated duplicate cancer at surgery; death or recurrence before the day of postoperative computed tomography (CT) at 4 months post-surgery; missing data. An opt‐out consent process was performed because of the retrospective nature of the study. This study was approved by the research ethics committee of the National Cancer Center (2019-075) in accordance with the Declaration of Helsinki. Informed consent was obtained through an opt‐out consent process.
Assessment of patient characteristics
We obtained the following information from medical records: age; sex; NAC; histological tumor type; Charlson comorbidity index (CCI) [10]; pathological stage according to UICC-TNM classification, 7th edition; postoperative complications, including pneumonia and anastomotic leakage (≥ II according to Japan Clinical Oncology Group postoperative complications criteria, in line with the Clavien–Dindo classification [11]); and length of hospital stay (LOS).
Assessment of physical function, nutrition, and inflammation
Isometric quadriceps muscle strength (QS; IsoForce GT-330, OG GIKEN, Japan) [12] and 4-m usual gait speed (UGS) [13] were collected as physical function indicators. For QS analysis, the side with a greater muscle strength before surgery was analyzed. Body mass index (BMI) and prognostic nutrition index (PNI) [14] were collected as nutritional indicators. C-reactive protein (CRP) [15] and neutrophil–lymphocyte ratio (NLR) [16] were collected as inflammation indicators. All factors were measured within 3 months before surgery and at first month after surgery. Percentage changes in QS, UGS, BMI, and PNI were calculated as follows: ([postoperative – preoperative] ÷ preoperative value) × 100. The postoperative change in CRP and NLR was measured as a bi-variable; postoperative CRP and NLR were defined by cutoff points of 0.5 mg/dL and 3.5, respectively [15, 16].
Assessment of percentage change in SMI
The skeletal muscle mass index (SMI) [17] was calculated from CT images at the level of L3. CT was performed twice within 3 months, prior to and 4 ± 2 months after surgery. Regarding preoperative CT images, CT images after NAC were used if the patient was treated with NAC. The cross-sectional area (Hounsfield unit, –29 to 150) on CT images was measured in the skeletal muscle area using SliceOmatic (Imagelabo, Canada). The percentage change in SMI 4 months after surgery (SMI%) was calculated as follows: ([postoperative – preoperative] ÷ preoperative value) × 100.
Statistics
Descriptive statistics are presented as number of patients, mean ± standard deviation, and median (1st–3rd quartile). SMI, QS, UGS, BMI, PNI, CRP, and NLR were compared before and after surgery by a paired t test or Wilcoxon signed-rank test to confirm postoperative changes. Variables without differences pre- and post-surgery were excluded from the analysis. The correlations of all factors were analyzed with the Spearman’s rank correlation coefficient. Associations between SMI% and postoperative factors were analyzed by simple linear regression. Multiple linear regression was performed using the forced entry method. Explanatory variables were potential factors with a p < 0.2 in the univariate analysis. Subsequently, age, sex, preoperative SMI [7], pT, pN [6], histological type [18], CCI [10], and NAC [19] were selected as potential preoperative confounding variables. The characteristics of the significant postoperative factors in the multiple linear regression were analyzed using a one-way analysis of variance or χ2 test. Statistical significance was considered as a two-tailed p value < 0.05. All analyses were performed with SPSS version 26 (IBM Corp., Japan) for Windows.
Results
Patient characteristics
Of an initial 146 patients, 33 patients were excluded for the following reasons: 4 patients, R1–2 esophagectomy; 2 patients, duplicate cancer; 10 patients, death or recurrence; 17 patients, missing data. The final analysis included 113 patients (Table 1). There were differences between preoperative and postoperative values for SMI, UGS, QS, BMI, and CRP (Table 2). The mean SMI% was – 5.6% ± 8.5%; there was heterogeneity in individual SMI% values (Table 2; Fig. 1).
Impact of early postoperative factors on SMI
None of the variables showed high correlations (r > 0.7; Table 3). Multiple linear regression analysis showed that the percentage change in QS in the first month after surgery significantly impacted the SMI% independent of age, sex, preoperative SMI, pT, pN, histological type, CCI, NAC, and change in BMI (Table 4). The coefficient of determination (R2) of the model was 0.218.
Association of significant postoperative factors with other factors
All patients were divided into two groups with a median value (– 9.0%) of the percentage change in QS as the cutoff point. The early postoperative major decline in QS group (percentage change, ≤ – 9.0%) had significantly lower preoperative SMI%, a greater decline in UGS and PNI 1 month after surgery, and longer LOS than the minor decline in QS group (Table 5).
Discussion
We investigated the impacts of early postoperative factors on changes in skeletal muscle mass after curative esophagectomy in older patients aged ≥ 65 years with EC. The mean SMI% 4 months after esophagectomy was – 5.6%. As shown in the multiple regression analysis, early postoperative change in QS in the first month after esophagectomy impacted the change in SMI 4 months after esophagectomy, which was independent of age, sex, baseline SMI, tumor stage and type, comorbidity, and NAC.
To our knowledge, the present study is the first study to investigate the impact of early postoperative factors, such as physical function, nutrition, and inflammation, on changes in skeletal muscle mass after esophagectomy in older patients. Skeletal muscle mass is affected by irreversible factors, such as aging [4], sex [4], tumor stage [6], disease [4], and chemotherapy [19], as well as reversible factors, such as physical function [9], nutrition [20], and inflammation [20]. We found that early postoperative changes in QS impacted changes in skeletal muscle mass 4 months post-esophagectomy in a dose-dependent manner in older patients, which was independent of irreversible factors.
The mechanisms behind the impact of early changes in muscle strength on changes in skeletal muscle mass could include potential mechanical stress on muscle fibers. It is well known that changes in the mechanical stress on muscle fibers can impact muscle strength via neurological and metabolic mechanisms [21, 22]. In addition, long-term changes caused by mechanical stress on muscle fibers could alter muscle mass due to an alteration in the protein synthesis and degradation balance, resulting in muscle atrophy or hypertrophy [23]. In a previous study, during a short-term bed rest period of 1–3 weeks, changes in muscle strength were reported to be faster and greater than changes in skeletal muscle mass [24, 25]. Furthermore, previous large longitudinal cohort studies suggested that changes in muscle strength preceded changes in skeletal muscle mass in community-dwelling older adults [9, 26]. In the present study, the early postoperative major decline in QS group had a greater decline in UGS and longer LOS than the minor decline in QS group. UGS and LOS are reportedly associated with physical activity [27, 28]. Hence, the early postoperative change in QS may have been influenced by postoperative physical activity during the hospital stay and after discharge, which could have led to subsequent changes in SMI.
In the present, the early postoperative massive decline in QS was characterized by lower preoperative SMI and a greater decline in PNI in the first month after surgery compared to the group of minor decline in QS. Older patients with preoperative vulnerabilities, such as low SMI, may have low resilience for muscle strength recovery [4]. Regarding the association between muscle strength and nutrition, malnutrition, such as vitamin E, carotenoids, and selenium deficiencies, was associated with lower muscle strength in a previous study [29]. Therefore, our findings, which indicate that early postoperative massive decline in QS is associated with preoperative SMI and change in PNI, are consistent with previous reports. In a recent meta-analysis, comprehensive rehabilitation, such as exercise therapy with protein supplementation, for older adults with risk of sarcopenia and frailty was reported to improve muscle strength and skeletal muscle mass [30]. In addition, the mechanism of the prognostic impact of postoperative loss of SMI was suggested to be associated with progression of frailty [7]. Considering our findings and previous studies, we hypothesize that in older patients with EC, continuous postoperative comprehensive rehabilitation, including exercise and nutrition therapy after perioperative rehabilitation, may prevent the loss of skeletal muscle mass and progression of frailty after esophagectomy by improving muscle strength.
There are several limitations to the present study. First, this study was a retrospective cohort study conducted at a single center. To confirm our results with validity and generalizability, prospective multicenter studies conducted with larger sample sizes are needed. Second, potential postoperative factors that had a strong impact may not have been included in the analysis. We were unable to assess physical activity, dietary intake, or cognitive and social function because of the retrospective nature of the study. Furthermore, although there was heterogeneity in the individual SMI% (Fig. 1), the coefficient of determination (R2) for the multiple regression analysis was 0.218; this indicated that the fit of the model was poor. Hence, interpretation of the results must be performed carefully and with consideration of the limitations.
In conclusion, in 113 patients aged 65 years or older with EC, the change in QS in the first month after esophagectomy impacted changes in SMI 4 months after surgery in a dose-dependent relationship, which was independent of irreversible factors such as age, sex, preoperative SMI, tumor stage, histological type, comorbidity, and NAC.
Data availability
Due to the nature of the retrospective study, participants of this study did not agree for their data to be shared publicly. Thus, data is not available publicly.
References
Watanabe M, Toh Y, Ishihara R et al (2022) Comprehensive registry of esophageal cancer in Japan, 2014. Esophagus 19(1):1–26
Cijs TM, Verhoef C, Steyerberg E et al (2010) Outcome of esophagectomy for cancer in elderly patients. Ann Thorac Surg 90(3):900–907
Tapias LF, Muniappan A, Wright CD et al (2013) Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg 95(5):1741–1748
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31
Williams GR, Dunne RF, Giri S et al (2021) Sarcopenia in the older adult with cancer. J Clin Oncol 39(19):2068–2078
Takahashi K, Watanabe M, Kozuki R et al (2019) Prognostic significance of skeletal muscle loss during early postoperative period in elderly patients with esophageal cancer. Ann Surg Oncol 26(11):3727–3735
Harada T, Tatematsu N, Ueno J et al (2022) Prognostic impact of postoperative loss of skeletal muscle mass in patients aged 70 years or older with esophageal cancer. Ann Surg Oncol 29(9):5638–5645
Dos Santos L, Cyrino ES, Antunes M et al (2017) Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle 8(2):245–250
Auyeung TW, Lee SWJ, Leung J et al (2014) Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr Gerontol Int 14:76–84
Kubo Y, Tanaka K, Yamasaki M et al (2021) Influences of the Charlson comorbidity index and nutrition status on prognosis after esophageal cancer surgery. Ann Surg Oncol 28(12):7173–7182
Katayama H, Kurokawa Y, Nakamura K et al (2016) Extended Clavien-Dindo classification of surgical complications: Japan clinical oncology group postoperative complications criteria. Surg Today 46(6):668–685
Tatematsu N, Hasegawa S, Tanaka E et al (2013) Impact of oesophagectomy on physical fitness and health-related quality of life in patients with oesophageal cancer. Eur J Cancer Care 22(3):308–313
Guralnik JM, Simonsick EM, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49(2):85–94
Onodera T et al (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. J Jpn Surg Soc 85:1001–1005
Huang Y, Feng JF, Liu JS et al (2015) Prognostic role of serum C-reactive protein in esophageal cancer: a systematic review and meta-analysis. Ther Clin Risk Manag 11:89–94
Pirozzolo G, Gisbertz SS, Castoro C et al (2019) Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis 11(7):3136–3145
Mourtzakis M, Prado CM, Lieffers JR et al (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006
Stein HJ, Siewert JR (2004) Improved prognosis of resected esophageal cancer. World J Surg 28(6):520–525
Kamitani N, Migita K, Matsumoto S et al (2019) Association of skeletal muscle loss with the long-term outcomes of esophageal cancer patients treated with neoadjuvant chemotherapy. Surg Today 49(12):1022–1028
Yoon HG, Oh D, Ahn YC et al (2020) Prognostic impact of sarcopenia and skeletal muscle loss during neoadjuvant chemoradiotherapy in esophageal cancer. Cancers 12(4):925. https://doi.org/10.3390/cancers12040925
Manini TM, Clark BC (2012) Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 67(1):28–40
Foong YC, Chherawala N, Aitken D et al (2016) Accelerometer-determined physical activity, muscle mass, and leg strength in community-dwelling older adults. J Cachexia Sarcopenia Muscle 7(3):275–283
Gao Y, Arfat Y, Wang H et al (2018) Muscle atrophy induced by mechanical unloading: mechanisms and potential countermeasures. Front Physiol 9:235. https://doi.org/10.3389/fphys.2018.00235
Marusic U, Narici M, Simunic B et al (2021) Nonuniform loss of muscle strength and atrophy during bed rest: a systematic review. J Appl Physiol 131(1):194–206
McDonnell AC, Eiken O, Frings-Meuthen P et al (2019) The LunHab project: muscle and bone alterations in male participants following a 10day lunar habitat simulation. Exp Physiol 104(8):1250–1261
Westbury LD, Syddall HE, Fuggle NR et al (2020) Long-term rates of change in musculoskeletal aging and body composition: findings from the health, aging and body composition study. Calcif Tissue Int 106(6):616–624
Spartano NL, Lyass A, Larson MG et al (2019) Objective physical activity and physical performance in middle-aged and older adults. Exp Gerontol 119:203–211
Morikawa A, Naito T, Sugiyama M et al (2018) Impact of cancer cachexia on hospitalization-associated physical inactivity in elderly patients with advanced non-small-cell lung cancer. Asia-Pac J Oncol Nurs 5(4):377–382
Robinson S, Granic A, Sayer AA (2019) Nutrition and muscle strength, as the key component of sarcopenia: an overview of current evidence. Nutrients 11(12):2942. https://doi.org/10.3390/nu11122942
Liao CD, Chen HC, Huang SW et al (2019) The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: a systematic review and meta-regression analysis of randomized trials. Nutrients 11(8):1713. https://doi.org/10.3390/nu11081713
Acknowledgements
The authors thank the members of the Department of Rehabilitation medicine, Esophageal Surgery, for their support; this research would not have been possible without their cooperation. We thank Georgia Lenihan-Geels, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Tsuyoshi. H. and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: TH, NT, TF, TT. Data collection and analysis: TH, NT, JU, YK, NK, HF, TF. Writing—reviewing and editing: TH, NT, TF, TF, NH, AW, AI, TT. Supervision: TF, TT.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest with regard to the research, authorship, or publication of this article.
Ethical approval
This study was approved by the research ethics committee of the National Cancer Center (2019–075) in accordance with the Declaration of Helsinki and was started thereafter.
Informed consent
An opt-out consent process was performed due to the retrospective nature of the study in all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harada, T., Tatematsu, N., Ueno, J. et al. Impact of early postoperative factors on changes in skeletal muscle mass after esophagectomy in older patients with esophageal cancer. Eur Geriatr Med 14, 203–210 (2023). https://doi.org/10.1007/s41999-022-00735-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-022-00735-0