Abstract
A DFT method was used to enlighten the reaction mechanism between dimethylacetylendicarboxylate (DMAD) and acetylacetone (ACAC) in the presence of triphenylarsine (TPA) as an efficient catalyst. Different paths of mechanism and transition states with possible intermediates were proposed and evaluated thermodynamically and kinetically. The results showed that the reaction starts with a nucleophilic attack by the TPA on the DMAD, and then it was followed by other four steps. For all of the intermediates, Z conformer was more favorable than E conformer, and also the trans configuration of the product was more favorable than of cis configuration. Enol form of ACAC was more preferred than keto form in step4, but keto form was favorable for the other steps. For this reason, the reaction mechanism couldn’t proceed through the enol-form, and the mechanism regarding keto-form of ACAC was the right mechanism. There were two possibilities for the proton-transferring step4 which lead to different products. Trans di (methoxycarbonyl)-3,3-diacetylcyclopropane product 3, which was the result of 1, 3 non-linear proton-transfer arrangement and (Z)-Alkene dimethyl 2-(pentane-2,4-dione) fumarate (product 4) which was produced by 1, 2 non-linear proton-transferring arrangement. Thermodynamic parameters exhibited that Z-Alkene is more stable than cyclopropane product, but cyclopropane product was more preferred kinetically (Ea trans cyclopropane = 44.75 kcal mol−1, Ea Alkene = 66.75 kcal mol−1).
Similar content being viewed by others
1 Introduction
Cyclopropanes moieties are used as important intermediates in the synthesis of more functionalized cycloalkanes and acyclic compounds. There are natural and synthetic cyclopropane compounds with biological activities such as antibacterial, antiviral, and antitumoral and also enzyme inhibition. In the structure of some drugs like Odanacatib, Singulair, and ciprofloxacin, cyclopropane groups are available. Cyclopropane includes in some important process such as cyclopropanation, ring-opening, cyclization or ring expansion. So these compounds are important building blocks in organic compounds synthesis. Cyclopropane moiety with the electron-donating or the electron-withdrawing groups is importance intermediates in organic chemistry because these compounds imply a mild condition to the important synthesis of 1,3-zwitterionic intermediates which participate in various reactions. Stereo and enantioselective synthesis is an important subject to researchers. Commonly three methods are used to synthesis a stereoselective cyclopropane, and they are as follow: (1) transition-metal catalyzed the decomposition of diazo compounds; (2) Michael-initiated ring closure (MIRC) and; (3) the cyclopropanation of olefins with halomethyl metal reagents [1,2,3,4,5,6,7,8]. The stereoselective synthesis of di(methoxycarbonyl)-3,3-diacetylcyclopropane via the reaction between dimethylacetylendicarboxylate (DMAD) and acetylacetone (ACAC)was reported catalyzed by triphenylarsine (TPA) (Scheme 1) [9]. We have previously studied the kinetics of this reaction and compared to the presence of triphenylphosphine (TPP) as a catalyst [10]. TPA reacted as a stronger nucleophile and a catalyst, resulting in the fourth step of the reaction (step4, k4, a proton transfer process) being recognized as the RDS. The reaction followed by second-order kinetics. The proposed mechanism was adapted in accord with the experimental results and the steady-state assumption. The results showed that the reaction rate decreases in the presence of DBM, which participates in the second step (step2), compared to ACAC when it is present as another 1,3-dicarbonyl compound (structural effect). Also, the partial order of the reaction concerning the 1,3-dicarbonyl compound was one. As a significant result, not only did a change in the structure of one of the reactants (TPA instead of TPP) create a different product, but also the kinetics and reaction mechanism changed. Also, the ΔH‡ term was much more significant than the TΔS‡ term, so the reaction was enthalpy-controlled. Detection of intermediates and types of probable forms with experimental techniques was not possible. It is also important to calculate thermodynamic and kinetic data to confirm the reaction mechanism and provides comprehensive information on each mechanism step. For this purpose, the reaction is studied kinetically and thermodynamically in the presence of triphenylarsine as a catalyst (TPA) using computational methods (Scheme 1) [11, 12]. Different paths of mechanism and transition states with possible intermediates are proposed. We hope that these details will provide comprehensive information on the kinetics and mechanism of this reaction and modify existing methods.
2 Computational methods
All quantum chemical calculations including geometry optimization, normal mode vibrational frequencies calculations of the species (1, 2, 3, I1, I2, I3, I4, P) in the reaction path was done using DFT method at B3LYP/6-31 + G(2d,p) [13,14,15] level of theory. Frequency calculations showed that TS coordination is a saddle point because of only one imaginary frequency and other species (with real frequency) are in a real minimum. The intrinsic reaction coordinates (IRC) [16] method was used to determine the TS structure for each step. All calculations were done using the Gaussian 09 program [17].
3 Results and discussion
The proposed mechanism is shown in Scheme 2. As can be seen, the mechanism involves five steps:
-
Step1 Nucleophilic attack between of TPA to DMAD and I1 formation.
-
Step2 I1 protonation by ACAC and production of I2 and ACAC−.
-
Step3 I3 production by the reaction of I2 and ACAC−, as two ions (I2 and ACAC−), results in I3.
-
Step4 1, 3 proton transfer in I3.
-
Step5 Cyclopropane ring (product) formation.
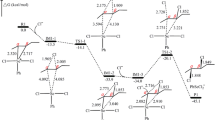
At the first step, the product (I1) is generated as a zwitterionic intermediate which can be either in E or Z forms (Fig. 1). Therefore, there are two states for each step (E or Z form) that are shown in Scheme S1 to S5 [Supplementary Information (SI)]. Also, the same challenge is for the remaining steps, therefore to identify the right conformation of the product species in the mechanism the potential energy profile investigated. For this reason in both E and Z forms, of each step (step1, step2, step3, step4, and step5) the distance between the two reactant atoms leading to a stable bond scanned, and the transition state for each step determined. The related potential energy profiles are shown in Figure S1 to S10 for each step. The E form of I1 has a bigger energy barrier then Z form, so the Z form of I1 is preferred. It should be mentioned that there are two transition states to reach E form of I1, first I1a(E) formed through TS1a and then I1E formation happened through TS1b. The total energy barrier for this process is 10.10 kcal mol−1, but for Z formation we have just one TS with an energy barrier of 7.5 kcal mol−1. So Z formation is easier than E. The potential energy profiles for the formation of cis and trans during the reaction path, involving six and five TS, respectively, cyclopropane obtained in the gas phase at the B3LYP/6-31 + G(2d,p) level of theory are presented in Fig. 1.
The cis and trans conformations of the final product 3 also investigated in the gas phase at B3LYP/6-31 + G(2d,p) level of theory. Some selected geometry parameters for each step contributors are given in Table S1.
The kinetic and thermodynamic parameters for E and Z forms of the species in the reaction mechanism are presented in Table 1. The Z and E forms comparison demonstrates that activation energy and it’s parameters (ΔG‡, ΔH‡, and ΔS‡) are in favor of Z form than E, so we have a bigger rate constant for Z than E. Thus Z form is preferred.
According to thermodynamic parameters, ΔG° value indicates that all steps are exothermic except step1 and step4 in both Z and E forms. Step4 has the minimum rate constant and maximum energy of activation, so this step is the RDS. Moreover, step5 is the fastest step among others, according to kinetic parameters reported in this Table. The thermodynamic parameters for the optimized E and Z structures of the all intermediates (I1, I2, I3, I4) and product (3) calculated and the results are presented in Table S2. As can be understood, Z form is preferred for all intermediates and product 4 and also a trans form of product 3.
Table 2 shows the thermodynamic parameters of trans and cis forms of the product 3, the more negative value of the thermodynamic parameters for trans form ensure us that Z form of the product 3 is favorable thermodynamically, of course, product 4 (Z form) will be discussed next.
The thermodynamic energy of 1, 2 intermolecular proton shift for the Z form of I3 in step4 was calculated and the energy profile is shown in Figure S11. The transition state was obtained by scanning the bond length between H6 and C2 (Scheme 2). The results exhibited that in this process, the proton transferring (H6) and the leaving group (triphenylarsine, catalyst) is concerted, and product 4 can be produced in this step. To comprise the energy barrier of product 4 (the result of 1, 2 H-shifted) and product 3 (the result of 1, 3 proton shift) it’s cleared that product 4 with the energy barrier of 66.75 kcal mol−1 is more stable than product 3 (with the energy barrier of 44.75), so product 4 is thermodynamically-controlled.
3.1 Keto and enol forms of acetylacetone
If we look at carefully to the reaction mechanism in Scheme 2. It will be understood that the exception of step1, ACAC moiety has been contributed to other steps (containing step2, step3, step4).
The question is, which form of ACAC, enol-or-keto form, can be participated in the reaction mechanism, particular step2, step3, and step4. To answer this, keto-enol tautomerization of acetylacetone (ACAC) was regarded to find out the right tautomer in the reaction mechanism. For this reason, the geometry optimization of both forms carried out using B3LYP/6-31 + G (2d,p) level of theory. The thermodynamic parameters (G°, H°, S°) of keto and enol forms of ACAC and ACAC−, respectively, are shown in Table S3. The details can be explained. Clearly; the recorded result in Table S3 indicated that keto form could exist as the two enol form; Enol-ACAC1 and Enol-ACAC2, rows 2 and 3, respectively. It was realized that Enol-ACAC1 is more stable.
When keto form of ACAC losses a proton, it converted to ACAC− form. This form can be found at the five forms; keto-ACAC1−, keto-ACAC2−, Enol-ACAC1−, Enol-ACAC2− and Enol-ACAC3−, rows 4, 5, 6, 7 and 8, respectively. The recorded results in Table S3 exhibited that most stable form among various ACAC− form is keto-ACAC1−, (G°, row 4) and among ACAC form is Enol-ACAC1−(G°, row 2). The reaction mechanism, regarding enol form of ACAC for steps 2, 3 and 4 is shown in Scheme 3, respectively.
In the proposed mechanism [9], in Scheme 2 keto form of ACAC starts the reaction, but theoretical studies on the keto and enol forms of ACAC confirmed that enol form is more stable than keto energetically. Therefore to examine exactly which form of ACAC take place in the reaction, the energy profile of the reaction in the case of enol form investigated at B3LYP/6-31 + G(2d,p) of theory. As shown in the mechanism, in the case of enol form of ACAC (enol-ACAC1) two pathways are possible for proton donation to I1, Scheme 3-pathway A, and Scheme 4-pathway B.
3.2 Reaction mechanism investigation in the presence of enol or keto form
3.2.1 Pathway A
Scheme 3 demonstrates the reaction mechanism in the case of HA (H1) transferring (subscript A refers to the pathway). To obtain the TS structure, the distance between C3–H1 was scanned (step2). The potential energy profile of the step2 is shown in Figure S12. Optimization of I1 and ACAC shows that unusually ph3As should be separated from DMAD, then after proton transferring (H1) the ph3As will be attached again, and I2 generates (by passing through TS2). The optimized geometries of the species in step2 are illustrated in Fig. 2. Then, in step3 ACAC2− attacks to I2 (Scheme 2) and C3–O bond will be formed between ACAC2− and I2 for unfavorable product (I3) with higher energy barrier instead of C3–C4 bond formation, for deserve Trans-product (3), shown in Scheme 2, so the remaining steps will not proceed, and this pathway should be discarded.
3.2.2 Pathway B
Scheme 4 exhibits the mechanism in the case of HB transferring (H2). Herein, in step2 the potential energy profile demonstrated, the proton (H2) transferring for C3–H2 bond formation and C3–C4 bond formation between ACAC− and I2 is concerted and appears as one step, and step3 did not exist as shown in Fig. 3. The potential energy profile for transferring H2 to C3 is shown in Figure S13.
The energy barrier for the concerted reaction in the step2 of pathway B that eliminated step3 to form (I3) is more than the sum of energy barrier in step2 and step3 in the case of keto form of ACAC (see step2 and step3 in Scheme 2). The geometry of optimized structures for the species in step2enol (I1 + ACACenol, TS2, I3) is shown in Fig. 3. Following step (step4) is proton-transferring from O3 to C5. The distance between H1 and C5 is so much for proton transfer. There is a geometrical restriction for this step, so the molecule changes it’s carbonyl direction to form I3a (Fig. 4) by an energy barrier of 60 kcal mol−1 to simplify 1, 5 proton transfer within the energy barrier of 5 kcal mol−1 as shown in Figure S14. This process (1, 5 proton transfer) consists of two steps with a total energy barrier of 65 kcal mol−1. To sum up, the energy barrier for the reaction in pathway B is too high (90 kcal mol−1), so the reaction can’t proceed from the enol form. According to the results, H2 transferring is possible by the concerted reaction and lead to the product by passing through a difficult route, so the reaction mechanism regarding keto form of ACAC is the right mechanism.
3.3 Tautomerization of I3
Another possibility for the reaction is the conversion of keto form of I3 to enol, and the 1, 5 proton-shift process occurs to make I4 and subsequently the trans-product, Scheme 5. The energy barrier for the keto-enol conversion (stepketo-enol) is too high, but the proton transfer process needs a little energy to proceed (see Figure S15 and S16).
Kinetics and thermodynamic parameters for these steps are reported in Table 3. According to the results, keto-enol conversion step has the highest energy barrier (61.2 kcal mol−1) and 1, 5 proton-transfer in step4 has the less energy barrier (5.2 kcal mol−1) among other steps in the case of enol form. So step keto-enol can be regarded as RDS. The energy barrier for this route is too high so the reaction can’t proceed through this mechanism.
4 Conclusion
A Theoretical kinetics investigation carried out at the B3LYP/6-31 + G (2d,p) level of theory to determine the mechanism, RDS and the real path in the reaction among DMAD, triphenylarsine, and acetylacetone (ACAC) for the generation of Trans-cyclopropane product (3).
The results are summarized as follow:
-
1.
Reaction proceeds in five steps and step4 are RDS.
-
2.
The overall reaction is exothermic and a spontaneous process.
-
3.
The intermediates in the reaction path prefer Z form than E.
-
4.
The reaction is kinetically-controlled, and the product is trans cyclopropane
-
5.
I1 formation in the step1 can be generated through the two reaction paths. The reaction path leads to Z conformer has one transition state with the energy barrier of (7.5 kcal mol−1), and E conformer needs a path through the two transition states with the energy barrier of 10.10 kcal mol−1. It seems that the stereoselectivity of the reaction originates from step1.
-
6.
Consideration of keto-enol tautomerization of ACAC in the reaction mechanism (for five steps) exhibited that in step4 enol form of ACAC is only preferred, and in the other steps keto form is favorable. Therefore, theoretical calculations indicated that examining of enol form instead of keto form of acetylacetone (ACAC) during the reaction path containing pathway A and tautomerization of some intermediates as enol forms proceed the reaction through a difficult rout with highest total energy barrier, so the reaction can’t proceed from the enol form and the mechanism regarding keto form of ACAC is the right mechanism.
-
7.
In the step4, there are two possibilities for the proton-transfer (a) 1, 2 proton-transfer and (b) 1, 3 proton-transfer. In the case of “a”, the product is an alkene, and in “b” case, cyclopropane compound will be formed. The results exhibited that alkene thermodynamically is more stable than cyclopropane product but, the big energy barrier makes it unfavorable kinetically.
5 Supplementary Information (SI)
Scheme of reaction for each step (E or Z form) and the potential energy profile included along with some tables in the supplementary information.
References
Lebel H, Marcoux JF, Molinaro C, Charette AB (2003) Stereoselective cyclopropanation reactions. Chem Rev 103:977
Neset S, Hope H, Undheim K (1997) Stereoselective synthesis of cyclopropane-1, 2-bis (glycine) derivatives. Tetrahedron 53:10459
Shen X, Liu Q, Zhang W, Hu J (2016) Stereoselective synthesis of (sulfonimidoyl) cyclopropanes with (R)-PhSO (NTs) CH2Cl and α, β-unsaturated weinreb amides: tuning the of selectivity between C–Cl and C–S bond cleavage. Eur J Org Chem 5:906
Watson ID, Ritter S, Toste FD (2009) Asymmetric synthesis of medium-sized rings by intramolecular Au(I)-catalyzed cyclopropanation. J Am Chem Soc 131:2056
Xie H, Zu L, Li H, Wang J, Wang W (2007) Organocatalytic enantioselective cascade Michael-alkylation reactions: Synthesis of chiral cyclopropanes and investigation of unexpected organocatalyzed stereoselective ring opening of cyclopropanes. J Am Chem Soc 129:10886
Tong W, Wang C, Zhao W, Chen J, Wu X, Zhang M, Deng H, Shao M, Ren Z, Cao W (2009) Facile, highly stereoselective synthesis of cyclopropyl benzoimidazoles via cyclopropanation of olefin with arsonium ylides. Synth Commun 39:3471
Macaev FZ, Malkov AV (2006) Use of monoterpenes, 3-carene and 2-carene, as synthons in the stereoselective synthesis of 2, 2-dimethyl-1, 3-disubstituted cyclopropanes. Tetrahedron 62:9
Zhao YH, Zheng CW, Zhao G, Cao WG (2008) Highly enantioselective tandem cyclopropanation/Wittig reaction of α, β-unsaturated aldehydes with arsonium ylides catalyzed by recyclable dendritic catalyst. Tetrahedron Asymmetry 19:701
Maghsoodlou MT, Khorassani SM, Heydari R, Charati FR, Hazeri N, Lashkari M, Rostamizadeh M, Marandi G, Sobolev A, Makha M (2009) Highly stereoselective construction of functionalized cyclopropanes from the reaction between acetylenic esters and C–H acids in the presence of triphenylarsine. Tetrahedron Lett 50:4439
Darijani M, Habibi-Khorassani SM, Shahraki M (2018) Effect of reactivity on kinetics and a mechanistic investigation of the reaction between dimethyl acetylenedicarboxylate and 1, 3-dicarbonyl compounds in the presence of a catalyst: a spectrophotometric approach. Prog React Kinet Mech 43:79
Darijani M, Habibi-Khorassani SM, Shahraki M (2015) A thermodynamic and kinetic insight into the pathways leading to a highly functionalized ketenimine: a computational study. Int J Chem Kinet Mech 47:751
Asheri O, Habibi-Khorassani SM, Shahraki M (2018) A study on the kinetics and mechanism of the one-pot formation of 3, 4, 5-substituted furan-2 (5H)-ones in the presence of lactic acid: effect of different substituents. Prog React Kinet Mech 43:286
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Becke AD (1996) Density-functional thermochemistry IV. A new dynamical correlation functional and implications for exact-exchange mixing. J Chem Phys 104:1040
Montgomery JA Jr, Ochterski JW, Petersson GA (1994) A complete basis set model chemistry. IV. An improved atomic pair natural orbital method. J Chem Phys 101:5900
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji (2010) H Gaussian 09, revision A01. Gaussian Inc, Wallingford
Acknowledgements
This work was supported by the University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Darijani, M., Shahraki, M. & Habibi-Khorassani, S.M. Kinetic study of stereoselective synthesis of highly functionalized cyclopropane catalyzed by triphenylarsine. SN Appl. Sci. 1, 553 (2019). https://doi.org/10.1007/s42452-019-0587-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0587-0