Abstract
Drought stress is an unavoidable challenge limiting plant production and quality. Stigmasterol is a potential compound for plant protection and improvement productivity under drought. Thus, the effects of using stigmasterol as exogenous treatment on improving growth and productivity of sunflower grown under drought were studied. A pot experiment was carried out at two summer seasons, using foliar treatment of stigmasterol 0, 100, 200, and 300 mg L−1 on sunflower plants under different irrigation levels 80% and 50% water irrigation requirement (WIR). Drought stress (50% WIR) provoked significant reductions in growth and yield components; the percentages of decrease in head diameter reached 26.55%, head circumference 26.05%, seed weight per plant 36.26%, and 100 seed weight 29.61%, via decreasing photosynthetic pigments and indole acetic acid while elevating hydrogen peroxide (H2O2), lipid peroxidation (MDA), membrane leakage, lipoxygenase activity, some antioxidant compounds, enzymes, and osmolytes. Stigmasterol has a promotive effect on growth and productivity of sunflower through improving photosynthetic pigments, indole acetic acid, non-enzymatic, enzymatic antioxidant, and osmolytes, while it decreased membrane leakage, H2O2, and MDA, thus, improving yield quality. Moreover, stigmasterol improves the economic importance of sunflower seed oil. About 200 mg L−1 of stigmasterol was the most effective concentration in improving yield parameters, as it causes 19.84% and 25.29% in seed weight per plant and 26.72% and 33.95% of 100 seed weight under 80% and 50% WIR, respectively. Stigmasterol improved growth and productivity of sunflower under normal water conditions and could overcome the reduced impact of drought by improving growth and development and different physiological attributes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Drought stress is one of the greatest abiotic stresses; it inhibits plant growth and productivity and decreases the production of more than 25% of agriculture worldwide. Additionally, drought negatively affects water relationships, absorption, and assimilation of nutrients, photosynthesis, and enzymatic activities (Bano et al. 2021). Prolonged drought might cause significant physiological and developmental alterations in plants (Hasan et al. 2018). Plants that experience drought exhibit a variety of metabolic disorders, including decreased water available in the soil and the necessity for water at evaporation sites, reactions to water status, and the genetic discrepancies of the plant species, thus reducing cell turgor and cell shrinkage (Xiong et al. 2020). Drought causes wilting, stomatal closure, and cell expansion decreases owing to decreases in cell water content, turgidity, and hydrostatic tissue pressure, leading to an inhibition of photosynthesis through alterations in chlorophyll content and damaging photosynthetic equipment, prominent reduction in transpiration, translocation, ion absorption, carbohydrates, nutrient metabolism, and growth promoters (Praba et al. 2009). Additionally, drought stress disrupts the equilibrium of cell homeostasis caused by an excessive production of reactive oxygen species (ROS) including hydrogen peroxide and superoxide radical which induces oxidative stress on proteins, membrane lipids, and interruption of DNA strands (Mittler 2020).
Sunflower (Helianthus annuus L.) is considered one of the most important oilseed crops, ranking third after soybean and peanut, among other oilseed crops in the world. (Thavaprakash et al. 2002). In Egypt, there is a huge shortage of vegetable oil production due to the enormous increase in the annual consumption of vegetable oils, which amounts to 2.66 million tons of oil, including 1820 tons of imported oil, in addition to 3790 tons of locally pressed oil seeds, leaving a gap of 97–98% (Adeleke and Babalola 2020). Approximately half of this amount is produced mainly from cotton seeds, and the other half is extracted from sunflower oilseeds in addition to imported soybeans (Aswaq Financial Co. 2018). Egypt produces about 300–400,000 tons of oil from a variety of crops including sunflowers, canola, soybeans, cotton, flax, olives, oil palm, and some other crops. Nevertheless, while sunflower production per unit area in Egypt has lately increased, productivity remains low, making it critical to increase productivity (FAOSTAT 2022). Only 2% of the consumption demands of edible oil produced by extraction of oilseeds. The remaining 98% was covered by the imported oils, in particular soybean oil, sunflower oil, corn oil, and palm oil (El-Hamidi et al. 2020).
Brassinosteroids (BRs) are a group of steroid growth regulators and signaling molecules and widely distributed in plants; their presence has been confirmed in algae, mosses, and vascular plants (Zullo and Bajguz 2019 and Bajguz 2019). BRs are playing an important role in various physiological processes in developmental processes, such as cell elongation, division, and vascular differentiation (Kour et al. 2021), reproductive development, seed germination, flowering pollen generation, regulation of gene expression, maturation, and plant aging (Nolan et al. 2020). Furthermore, BRs significantly boost transpiration potentiality and raise protein, carbohydrates, and chlorophyll levels (Li and Hi 2020). Additionally, BRs participate in plant’s tolerance to a wide range of biotic and abiotic stresses, including temperature extremes, drought, salinity, and pathogen attacks (Gruszka 2013, Ahanger et al. 2018 and Li et al. 2020). Stigmasterol belongs to the brassinosteroid; it is an integral component of the lipid center of cellular membranes and is the precursor of numerous secondary metabolites involving plant steroid hormones, acting as transporters in acyl, sugar, and protein passage (Krishna 2003).
To the best of our knowledge, little research has looked at the impact of foliar stigmasterol spraying on morphological and physiological traits as well as yield attributes of stressed sunflower plants. Thus, the objective of the current study was to investigate the utility of stigmasterol (with different concentrations) as a promising plant development regulatory substance to improve sunflower tolerance to water deficiency via growth, activities of photosynthetic pigments, osmolytes, anti-oxidative enzyme activities, antioxidant and antioxidant compound contents, cell membrane stability, and H2O2 content, and in addition, to yield characteristics and the nutritional quality of the yielded seeds as well as fatty acid profile of the yielded sunflower seeds.
2 Materials and Methods
2.1 Plant Material and Growth Conditions
A pot test was conducted in two successive seasons of summer 2020 and 2021 (temp. 36–38/17–22 °C, day/night, light intensity; 300 μmol m−2 s−1; and humidity; 60–70%) in the greenhouse of the National Research Center, Dokki, Giza, Egypt (30° 3′0″ N/31° 15′0″E), using sunflower (Helianthus annuus L.) cultivar Sakha 53. Seeds of sunflower were obtained from the Agricultural Research Center, Giza, Egypt. Stigmasterol used was supplied from Sigma–Aldrich. Healthy sunflower seeds were sterilized for 5 min using 5% sodium hypochlorite (NaOCl) and then washed using distilled water. Seeds were sown in pots (50 cm3) containing 7 kg of silty and sandy soil with a ratio of 1:1 to reduce compaction and improve drainage. The pots were divided into two major groups depending on irrigation with different water requirements (80% and 50% WIR), namely D0 (as control) and D1, respectively. Each of the previous groups was divided into four subgroups and foliar treated twice at 30 and 40 days after sowing with different concentrations of stigmasterol (0, 100, 200, and 300 mg L−1), namely Sts 0, Sts 1, Sts 2, and Sts 3, respectively. A preliminary germination experiment using different concentrations of stigmasterol (0.0, 50, 100, 150, 200, 250, 300, 250, and 400 mg L−1) was conducted. Then, the appropriate concentrations of Sts were chosen based on the results of growth characteristics. Each treatment consisted of five replicates distributed in a completely randomized design. Sunflower seeds were planted during the 13th of May of the two summer seasons of 2020 and 2021 and harvested on the 20th of August of the two seasons. Soil was fertilized with the mentioned fertilizers 3 days prior to planting in the following doses: (1) ammonium sulfate (20.5% N) at 10 g per pot; (2) super phosphate (15% P2O5) at a 4 g per pot dose; and (3) potassium sulfate (48% K2O) at a 2 g per pot dose. Soil water irrigation requirement was determined by saturating the soil in each pot with water, drainage for 48 h, and weighing. The soil water irrigation requirement (WIR) or field holding capacity was calculated. The soil water capacity was kept around 80 and 50% of its maximum level by weighing the pots and balancing the daily water loss. Sunflower seedlings were irrigated with the two levels of WIR twice weekly. Thinning was performed 15 days after sowing, and five plants were left in each pot. Pots of each plot are irrigated with one of the following water irrigation levels (80 and 50% WIR). Samples were collected after 50 days from sowing to determine the morphological measurements and chemical analysis. One plant per pot was left for yield determination. The morphological characteristics were plant height (cm), number of leaves and leaves area per plant, and fresh and dry weight of shoot (g per plant). In addition, root length (cm), root fresh and dry weight/plant (g) as well as relative water content (RWC%) of shoot were measured and expressed as percentage, according to the following equation (Alqurainy 2007).
When signs of full maturity stage, measurements for yield and its components were also recorded (head diameter (cm), head circumference (cm2), seed yield/plant (g), and 100 seeds weights (g).
2.2 Chemical Analysis
Determination of Photosynthetic Pigments. Chlorophyll and carotenoid contents in plant leaves were estimated using the method of Lichtenthaler and Buschmann (2001). Indole acetic acid content was extracted and estimated by the method of Larsen et al. (1962). The level of H2O2 was determined, according to Jana and Choudhuri (1981). According to Hodges et al. (1999), the level of lipid peroxidation was measured by determining the malondialdehyde (MDA) content. The electrolyte leakage was measured, according to the method recorded by Lutts et al. (1996). Lipoxygenase (EC 1.13.11.12) activity was evaluated, according to Doderer et al. (1992).
Assay of Antioxidant Enzyme Activities. Enzymes were extracted following the method of Chen and Wang (2006). Peroxidase (POX) (EC 1.11.1.7) activity was evaluated using the method of Kumar and Khan (1982). Superoxide dismutase (SOD, EC 1.15.1.1) activity was measured according to Chen and Wang (2006). Catalase (CAT) (EC 1.11.1.6) activity was determined, according to the method of Chen and Wang (2006). The level of ascorbate peroxidase (ASP) (EC 1.11.1.11) activity was estimated using Chen and Asada (1992) method. Nitrate reductase (NR) (EC 1.1.1) activity was assayed following the method of Foyer et al. (1998). Glutathione reductase enzyme (GR) (EC 1.6.4.2) activity was evaluated, according to the method of Foyer et al. (1995).
Assay of Non-Enzymatic Antioxidant Enzymes. Non-enzymatic antioxidants were extracted as the method described by Gonzalez et al. (2003). A known weight of the fresh samples (1 g) was homogenized with 85% cold methanol (50 mL v/v) for three times at 90 °C. The free radical scavenging activity by 2,2,-diphenyl-2-picryl-hydrazyl (DPPH) method was estimated, according to Sousa et al. (2007). Total phenol content was described by Gonzalez et al. (2003). Content of glutathione (non-protein SH group) was estimated, according to the method of Paradiso et al. (2008). α-Tocopherol content was assayed, according to the method of Jargar et al. (2012).
Assay of Compatible Solute Accumulation. Proline (Pro) content was determined by Kalsoom et al. (2016). Total soluble sugars (TSS) were estimated by the method of Homme et al. (1992). Total free amino acids (FAA) content was measured by the method of Sorrequieta et al. (2010).
2.2.1 Sunflower Yield and Its Components
The sunflower oil seeds were extracted, according to Das et al. (2002) by using Soxhlet apparatus. Total carbohydrates were evaluated, according to Albalasmeh et al. (2013). The total protein concentration of the supernatant was determined, according to the method described by Bradford (1976) with bovine serum albumin as a standard. Flavonoid compounds were determined, according to the method of Chang et al. (2002).
2.2.2 Determination of Fatty Acid
Fatty acids were extracted, according to Harbone (1984). The methylated samples were analyzed by using GLC apparatus in GVC pye Unicam gas-liquid chromatograph equipped with dual flame ionization detector and dual channel recorder.
2.3 Statistical Analysis
The values were subjected to the analysis of variance (ANOVA) for a factorial in a completely randomized design MSTAT-C (1988). The significant differences between means were compared at p ≤ 0.05 using Tukey’s HSD (honestly significant difference) test.
3 Results
3.1 Impact of Stigmasterol (Sts) and Drought (WIR) on Growth Criteria of Sunflower Plants
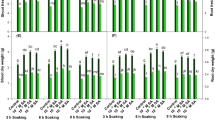
The effect of foliar spraying of stigmasterol treatment with different concentrations (0, 100, 200, and 300 mg L−1) on growth indices of sunflower plants grown under drought stress is presented in Table 1. It is obvious that decreasing water irrigation requirement from 80 to 50% WIR (D0 and D1) significantly decreased sunflower growth indices (plant height (cm), leaf number and area/plant, and shoot), while it increased significantly root length (cm), fresh and dry weight (g); and while non-significant decrease was recorded in relative water content (RWC%). The percentage of decreases reached to 14.4%, 10.6%, 18.5%, 20.6%, 15.9%, and 0.78%, in plant height, leaf number and area/plant and RWC%, with 24.1%, 24.2%, and 9.9% percentages of increases in root, length (g), fresh and dry weight (g), respectively Meanwhile, different Sts concentration caused significant gradual increases in the studied growth indices and RWC till 200 mg L−1, and then the increases in response to 300 mg L−1 were less than 200 mg L−1 but still more than the control plants. Moreover, data clearly show superiority of 200 mg L−1 Sts over the control plant and other used Sts treatments (Table 1). Furthermore, 200 mg L−1 could alleviate the negative effect of water stress by 38.6, 16.8, 122.7, 56.5, 30.7, 38.7, 38.7, 41.4, and 2.4 percentages of increases in the studied growth parameters, compared with untreated control (Table 1).
3.2 Impact of Stigmasterol (Sts) and Drought (WIR) on Photosynthetic Pigments and Endogenous Indole Acetic Acid of Sunflower Plants
The impact of stigmasterol with different concentrations on photosynthetic pigments constituents (Chl. a, Chl. b, carotenoids, and total pigments) Fig. 1, Chl. a/Chl. b, Fig. 2 as well as endogenous indole acetic acid (IAA) (Fig. 3) of sunflower plants grown under drought stress. Drought stress (50% WIR) significantly reduced Chl. a, Chl. b, carotenoids, and consequently total pigments as well as endogenous IAA, compared with control plants (80% WIR). Meanwhile the ratio of Chl. a/Chl. b was increased significantly by 50% WIR. The application of stigmasterol significantly increased those traits gradually to 200 mg L−1, then the increases in 300 mg L−1 were less compared to 200 mg L−1, while decreased Chl. a/Chl. b ratio. Sts treatment at 200 mg L−1 resulted in the highest increments in the above-mentioned traits, compared with other Sts levels, since it gave 96.6 and 79.4 (mg g−1 fresh weight) in Chl. a, 67.4 and 49.1 (mg g−1 fresh weight) in Chl. b, 32.4 and 32.0 (mg g−1 fresh weight) in carotenoids, 196.4 and 160.4 (mg g−1 fresh weight) in total pigments and 50.9 and 39.85 (μg/g fresh weight) in IAA; meanwhile in Chl. a/Chl. b ratio, 200 mg L−1 showed the least decrease 1.43 and 1.62 under normal and water stressed conditions, respectively.
Effect of different concentrations of stigmasterol (Sts) application on sunflower plants stressed by drought on photosynthetic pigment content (μg/100 g fresh weight). D0 (control) (80% water irrigation requirement WIR), D1 (50% water irrigation requirement WIR), Sts 0 (irrigation without stigmasterol treatment), Sts 1 (stigmasterol at 100 mg L−1), Sts 2 (stigmasterol at 200 mg L−1) and Sts 3 (stigmasterol at 300 mg L−1). Mean values within the same column for each trait with the same lower-case letter are not significantly different at p ≤ 0.05. Measurements were done at 50 days after sowing. (Each value represents the mean ± standard error (n = 3)
Effect of different concentrations of stigmasterol (Sts) application on sunflower plants stressed by drought on chlorophyll a/chlorophyll b ratio. D0 (control) (80% water irrigation requirement WIR), D1 (50 % water irrigation requirement WIR), Sts 0 (irrigation without stigmasterol treatment), Sts 1 (stigmasterol at 100 mg L−1), Sts 2 (stigmasterol at 200 mg L−1) and Sts 3 (stigmasterol at 300 mg L−1). Mean values within the same column for each trait with the same lower-case letter are not significantly different at p ≤ 0.05. Measurements were done at 50 days after sowing. (Each value represents the mean ± standard error (n = 3)
Effect of different concentrations of stigmasterol (Sts) application on sunflower plants stressed by drought on endogenous indole acetic acid (IAA) (μg/g fresh weight). D0 (control) (80% water irrigation requirement WIR), D1 (50% water irrigation requirement WIR), Sts 0 (irrigation without stigmasterol treatment), Sts 1 (stigmasterol at 100 mg L−1), Sts 2 (stigmasterol at 200 mg L−1) and Sts 3 (stigmasterol at 300 mg L−1). Mean values within the same column for each trait with the same lower-case letter are not significantly different at p ≤ 0.05. Measurements were done at 50 days after sowing. (Each value represents the mean ± standard error (n = 3)
3.3 Changes in Reactive Oxygen Species and Related Traits
During oxidative stress, reactive oxygen species (ROS) including hydrogen peroxide (H2O2) are generated, while lipid peroxidation is promoted by reactive oxygen species (ROS). The accumulation of ROS in plants damages almost all the cellular components, for example, cell membrane, lipids, pigments, and enzymes. ROS may cause cellular injury if not counteracted by antioxidant enzymes. Figure 4 shows the effect of stigmasterol (Sts) on hydrogen peroxide (H2O2), lipid peroxidation (MDA), and lipoxygenase (LOX) enzyme in sunflower plants grown under drought stress. Drought stress significantly increased oxidative damage parameters including H2O2 which resulted in a significant increase in lipid peroxidation (MDA), and lipoxygenase (LOX) enzyme. Sts application significantly decreased H2O2, which resulted in a significant reduction in MDA. Foliar Sts application at 200 mg L−1 resulted in best results, compared to other Sts levels. Stigmasterol concentration of 200 mg L−1 under normal irrigation resulted in a reduction in oxidative damage by 18.38% in H2O2 and consequently decreased MDA by 15.16%, and LOX by 14.75%, compared to control Sts. However, 200 mg L−1 Sts application under 50% WIR (drought stress) caused a reduction in oxidative damage by 34.19% in H2O2 and consequently decreased MDA by 28.13%, LOX by 31. 15%, compared to control Sts (Fig. 4).
a, b, and c Effect of different concentrations of stigmasterol (Sts) application on sunflower plants stressed by drought on hydrogen peroxide H2O2, malondialdehyde MDA, lipoxygenase LOX enzyme (nmole/g fresh weight). D0 (control) (80% water irrigation requirement WIR), D1 (50% water irrigation requirement WIR), Sts 0 (irrigation without stigmasterol treatment), Sts 1 (stigmasterol at 100 mg L−1), Sts 2 (stigmasterol at 200 mg L−1) and Sts 3 (stigmasterol at 300 mg L−1). Mean values within the same column for each trait with the same lower-case letter are not significantly different at p ≤ 0.05. Measurements were done at 28 days after sowing (Each value represents the mean ± standard error (n = 3)
It is clear from the obtained results in Fig. 5 that drought stress (50% WIR) causes a marked increase in membrane leakage of sunflower plants, compared with those of the reference controls (80% WIR). Meanwhile, application of stigmasterol with different levels caused significant decreases in membrane leakage of treated sunflower plants, compared with those of the reference controls. Sts at 200 mg L−1 under normal irrigation resulted in a reduction in ML by 17.83%, compared to control Sts. However, 200 mg L−1 Sts application under 50% WIR (drought stress) caused a reduction in oxidative damage by 28.13%, ML compared to control Sts (Fig. 5).
Effect of different concentrations of stigmasterol (Sts) application on sunflower plants stressed by drought on membrane leakage (ML%). D0 (control) (80% water irrigation requirement WIR), D1 (50% water irrigation requirement WIR), Sts 0 (irrigation without stigmasterol treatment), Sts 1 (stigmasterol at 100 mg L−1), Sts 2 (stigmasterol at 200 mg L−1) and Sts 3 (stigmasterol at 300 mg L−1). Mean values within the same column for each trait with the same lower-case letter are not significantly different at p ≤ 0.05. Measurements were done at 28 days after sowing (Each value represents the mean ± standard error (n = 3)
3.4 Changes in Enzymatic Antioxidants, Antioxidant Activities and Non-Enzymatic Antioxidants
3.4.1 Changes in Enzymatic Antioxidants
Drought stress significantly (p ≤ 5%) increased peroxidase (POX), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (ASP), nitrate reductase (NR), and glutathione reductase (GR) enzyme activities of sunflower plants, compared with well-watered plants (Table 2). Furthermore, distinct levels of stigmasterol (Sts) foliar application caused more significant increases in those studied antioxidant enzymes POX, SOD, CAT, ASP, NR, and GR enzymes, as compared with those untreated sunflower plants. Moreover, exogenous application of 200 mg L−1 Sts gave the highest increases in the above-mentioned enzymes, as compared with other levels of Sts (Table 2).
3.4.2 Changes in Antioxidant Activities and Non-Enzymatic Antioxidants
Antioxidant activities expressed by DPPH free radical scavenging activity and non-enzymatic antioxidants such as total phenolics (TPh), reduced glutathione GSH, and α-tocopherol. α-Toco were increased in drought-stressed sunflower plants, as compared with well-watered plants (Table 3). Furthermore, different concentrations of Sts caused more significant increases in DPPH, TPh, GSH, and α-Toco. Stigmasterol with concentration of 200 mg L−1 showed superiority in increasing the above-mentioned antioxidants, as compared with all other levels of Sts either under normal irrigation or drought-stress conditions. Thus, it is recommended to apply foliar Sts either under drought or no drought conditions and the best level of Sts application is 200 mg L−1(Table 3).
3.5 Changes in Compatible Solute Accumulation
Accumulation of compatible solutes is known to be a main way for plant defense and survival under abiotic stress, including drought and oxidative stress. Stigmasterol (Sts) impact on compatible solute accumulation (proline, Pro and total soluble sugars, TSS) in addition to free amino acids (FAA) contents of sunflower plants grown under drought stress are presented in Table 4. Decreasing irrigation water requirement from 80 to 50% WIR (drought stress) significantly increased Pro, TSS, and free amino acids over control plants (80%); the percentage of increase in Pro was 28.50%; in TSS, it was 28.48% and in FAA was 26.30%. Furthermore, exogenous application of Sts significantly further increased the accumulation of Pro, TSS, and FAA over untreated control plants. Sts at a rate of 200 mg L−1 gave the highest values, compared to all other Sts rates, so it is recommended to apply foliar Sts either under normal water irrigation or drought stress, and the best level of Sts application is 200 mg L−1 (Table 4).
3.6 Impact of Stigmasterol (Sts) and Drought (WIR) on Yield Traits of Sunflower Plants
Table 5 shows the effect of Sts on different yield and its components such as head diameter (cm), head circumference (cm), seed yield/plant (g), and 100 seed weight (g) of sunflower plants grown under drought stress. Relative to control, drought stress (D1) (50% WIR) significantly reduced yield components; the percentages of decreases were 26.55%, 26.05%, 36.26% and 29.61% of head diameter, head circumference, seed weight/plant, and 100 seed weight, respectively. While exogenous application of Sts significantly (100, 200, and 300 mg L−1) increased these traits gradually till 200 mg L−1, and then the increases in 300 mg L−1 were less compared to 200 mg L−1. Sts at 200 mg L−1 resulted in the highest values, compared to other treatments under control drought, and drought stress (D0) (80% WIR) gave the highest values (Table 5), as it caused 14.05% and 15.84% of head diameter, 13.79% and 16.58% of head circumference, 19.84% and 25.29% of seed weight and 26.72% and 33.95% of 100 seed weight under 80% and 50% WIR, respectively.
3.7 Changes in Nutritional Values of the Yielded Sunflower Seeds
The impacts of foliar treatment of sunflower plant with stigmasterol Sts different concentrations (0, 100, 200, and 300 mg L−1) on yielded seed quality including the nutritional value of the yielded seeds as oil%, carbohydrates CHO%, protein%, flavonoids%, and DPPH% are presented in Table 6 grown under normal irrigation and drought stress. Data clearly showed that decreasing water irrigation requirement from 80 to 50% WIR (drought stress) caused significant decreases in oil%, carbohydrates CHO%, and protein%, meanwhile increased significantly, as compared with those plants irrigated normally (80% WIR). Sts foliar treatment with different concentrations not only caused promotive effect on the above-mentioned indices of the yielded seeds in plants irrigated with 80% WIR, but also, alleviated the reduced effect of drought stress, compared with untreated controls. Sts with 200 mg L−1 was the most effective treatment, as it gave the highest increases in different studied parameters over the other treatments (Table 6).
3.8 Changes in Fatty Acid Contents
The constituents of fatty acids of the yielded sunflower seeds are depicted in Table 7. The fatty acids palmitic, stearic, palmitoleic, oleic, linolenic, and linoleic acid comprised most of the fatty acids in control plants; from unsaturated fatty acids, oleic acid was present in higher content followed by linoleic acid. While saturated fatty acid, palmitic was the dominant followed by stearic acid. Drought stress caused great decreases in total fatty acid as well as the contents of oleic, linoleic, and linolenic acids, whereas palmitic and stearic acids increased markedly.
Moreover, drought stress generated the fatty acid of behenic and lignoceric acids. Nonetheless, stigmasterol treatment with different levels caused marked increments in oleic, linoleic, and linolenic acids, compared with untreated controls either under normal or drought-stressed conditions (Table 7); while, palmitic and stearic acids were decreased relative to untreated control plants. Moreover, drought stress increased markedly total saturated fatty acids while it decreased total unsaturated fatty acids as well as the ratio of total unsaturated/ total saturated fatty acids TUS/TS. On the other hand, Sts treatments highly counterbalanced the effect of drought stress on fatty acid contents, as it improved them. Sts treatment augmented the obtained decrease in total fatty acid under drought stress. Moreover, Sts foliar application could alleviate the drought stress effect by decreasing total saturated fatty acid while increasing total unsaturated fatty acids and the ratio of total unsaturated/total saturated fatty acids TUS/TS. Moreover, the beneficial role of Sts treatment was either in unstressed or drought-stressed plant, the most effective effect recorded with 200 mg L−1 Sts.
4 Discussion
Drought stress is one of the environmental stressors that have a negative impact on sunflower plant growth, different metabolic activities, seed production, and nutritional quality. Stigmasterol exogenous treatments were evaluated to diminish the negative effects of drought stress on sunflower plants. Low water irrigation caused variations in a variety of biochemical, physiological, and molecular attributes that reduced nutrient availability and decreased plant growth and productivity (Tariq et al. 2019 and Kapoor et al. 2020).
According to the current study, sunflower plants under drought stress exhibited less growth features and yield components. These findings supported earlier studies on quinoa, wheat, flax, and moringa plants (Elewa et al. 2017, Bakry et al. 2019, Sadak et al. 2019 and Abd Elhamid et al. 2021).These reductions might be resulted from inhibiting the production of protein kinases, restricted cellular division, and expansion resulting in a reduction in apical growth (Kapoor et al. 2020 and Jing et al. 2022). Furthermore, water stress restricts the Calvin cycle’s ability to fix CO2 resulting in low fresh and dry matter production (Ozturk et al. 2021). These decreases might potentially be the result of pigment and protein alterations, oxidation of chloroplast, and lipids (Marcinska et al. 2013). The equilibrium among both leaves’ water supply and the rate of transpiration is indicated by the relative water content (RWC) of leaves. Decreased relative water content means less pressure, leading to reduced water available for regulator cell enlargement (Avramova et al. 2015). The lower leaf RWC may be due to reduced water availability, or the roots may become unable to reabsorb water lost through transpiration (Soltys-Kalinaet al. 2016).
Impaired photosynthesis caused via chloroplast degradation due to severe effects of drought stress from the significant signs of drought stress impacts (Sardans and Peñuelas 2012). These decreases are substantiated by previous studies on Moringa oleifera (Ezzo et al. 2018) and chickpea plant (Bakhoum et al. 2022). These reductions might be caused by oxidation of chloroplast lipids, modifications to the structures of pigment and protein molecule, or chlorophyll degradation by proteolytic enzymes like chlorophyllase (Marcinska et al. 2013) deterioration in chloroplast molecule and finally stomatal closure (Jomo et al. 2016).
Moreover, water deficiency decreased endogenous IAA of sunflower seedlings; these findings are consistent with those obtained by Abd Elhamid et al. (2021) on Moringa oleifera and Sadak and Ramadan (2021) on white lupine plants. Bano and Yasmeen (2010) reflected these decreases to the improvement in IAA oxidase activity, which would boost IAA degradation. Reduced IAA contents led to cell division and/or cell expansion decreases which in turn reduced growth rate.
Drought stress increased oxidative stress damage in terms of free radical accumulation such as H2O2 and lipoxygenase activity (LOX), leading to lipid peroxidation and membrane leakage. These free radicals react with lipid, protein, and DNA causing oxidative damage and disturb normal cellular functions (Gill and Tuteja 2010). These obtained data are supported with those obtained by Elkelish et al. (2019), Sadak and Bakhoum (2022), and Sadak (2022). ROS can permeate from sites which produce them to other sites of cells, harming delicate cell structures (Yin et al. 2015). A correlation between the increased H2O2 in leaves and the decreased chlorophyll content in wheat plants under salinity stress is also stated by Angelika et al. (2013). Membrane leakage ML is a crucial indicator of damaged cell membranes. Additionally, the loss of membrane integrity which resulted in increased ion leakiness and, as a consequence, an increase in membrane leakage that led to decreased membrane stability (Prabha and Negi 2014).
Under stress conditions, plants have internal mechanisms which reduce stress triggered by oxidative impacts by eliminating overproduced ROS (Kapoor et al. 2020). Antioxidants, both enzymatic and non-enzymatic, have a strong ability to scavenge excess ROS. It was intriguing to see how stigmasterol treatment increased the antioxidant system of the sunflower plant by raising enzyme activity and non-enzymatic component concentration (Sadak and Bakry 2020). O2•− radical is removed from numerous cellular locations, including the photosynthetic system, by superoxide dismutase (SOD), while H2O2 is scavenged by either CAT in the cytoplasm or by the ascorbate-glutathione cycle in chloroplast and mitochondria (Elkeilsh et al. 2019). Drought-stressed sunflower plants exhibited improved CAT and POX activities which involved quenching ROS, decreasing cellular damage and enhancing oxidative capacity to defend against stress (Sofy et al. 2021); while increases in SOD, POX, and CAT activities improve the elimination of excess ROS molecules, conferring higher stress resistance on sunflower plants. Increased antioxidant functioning prevents the vulnerability of photosynthetic electron transport to O2•− in the PSII reaction center, which otherwise results in irreversible oxidation of D1 protein (Borella et al. 2019).
Total phenols are an example of secondary metabolites that positively affect defense mechanisms by efficiently scavenging different ROS (Hasan et al. 2018). Total phenol contents of sunflower plant increased due to drought; these increases could be due to increased activity of enzymes known to be involved in their production (Bhattacharya et al. 2010). The same effect was obtained Abd Elhamid et al. (2021) who observed that drought increased phenol levels of Moringa olefera.
An earlier work by Sadak et al. (2019) discovered a rise in Pro and TSS contents of flax varieties under drought stress. Osmolytes found in the cytoplasm of plants have a basic effect on lowering cell osmotic potential and stabilizing cell turgor (Serraj and Sinclair 2002) without reducing actual water contents (Pathan et al. 2004). While proline (Pro) is one of the most significant osmolytes linked to plants experiencing oxidative stress and helps to stabilize subcellular structures (such as proteins and membranes) as well as scavenge free radicals, proline accumulation may be a way for plants to escape water stress (Huang et al. 2014). Herein, the increased production of proline and TSS might be the result of different biosynthetic and catabolic pathways being regulated. Furthermore, major cellular structures and their functionality could be safeguarded by adequate production of proline, TSS, and free amino acids. For example, proline protects the carboxylase activity of RuBisCO (Sivakumar et al. 2000). Proline is also a crucial component for controlling water absorption, preserving ROS homeostasis, and modulating communication (Mattioli et al. 2009).
Yield and its components showed significant losses in sunflower plants subjected to drought stress, while Sts treatment led to significant increases in yield components. Drought stress decreases plant growth, hence decreasing yield components. Therefore, the plant yield might result from integrating various metabolic processes. Thus, yield is affected by factors that disrupt these processes. In this study, the decreases in yield traits might be resulted from growth cessation, so plants are suffering from stress. The reduction in yield is associated with delayed flowering and decreasing flower number and pod set (Khan et al. 2016). The nutritional values of sunflower plants yielded seeds such as oil, total carbohydrates (CHO), and protein contents and were significantly reduced by drought, whereas their flavonoids contents and antioxidant activity increased. Similar findings were obtained in flax plant species by Sadak and Bakry (2020). Variations in a seed’s nutritional values such as oil, total carbohydrate, protein, and flavonoid contents are important since they are closely related to various biochemical and physiological metabolic processes (Anjum et al. 2003). Water stress lowered chlorophyll content of leaves, which in turn reduced photosynthetic activity.
Consequently, lowering carbohydrate deposit in mature leaves and, as a result, lowering the transit of carbohydrates from leaves to developing seeds (Elewa et al. 2017). To preserve energy and defend against stress, plants’ metabolic systems generally or the seeds they produce undergo changes that slow down the growth and development of seeds (Ragaey et al. 2022). In addition, seed oil and protein affect plant responses to environmental signals, particularly osmotic adjustment, either directly or indirectly (Singh and Sinha 2005). Meanwhile, the increased amount of flavonoid and antioxidant activity under drought conditions may reflect defense against stress conditions (Shimoi et al. 1996 and Geetha et al. 2003), since drought stress was accompanied by increased production of reactive oxygen species (Rezazadeh et al. 2012).
Sunflower oil’s fatty acid profile under drought stress was significantly countered by stigmasterol foliar treatments. The obtained decreases in the unsaturated fatty acids which are accompanied with increments in saturated fatty acid contents in sunflower oilseeds, due to decreasing water irrigation requirements (50%), are in accordance with the results of Hassan et al. (2021) on flax plant. According to Hajlaoui et al. (2009), the ability to adapt to drought stress may manifest as a potential decrease in desaturase activity of Schizochytrium limacinum. Malkit et al. (2002) also stated various plants might be shielded from oxidative stress via rebuilding membranes with less polyunsaturated fatty acids. Additionally, this less unsaturation degree hindered the elasticity of membrane (Zhao and Qin 2005; Upchurch 2008) and restricted permeability (Konova et al. 2009). Furthermore, Petcu et al. (2001) and Baldini et al. (2002) stated that drought significantly lowered oleic acid amount in sunflower oil, and they reflected this result to the enzyme ∆-9 desaturase that began to be active on the 8th day following flowering along with a rise in the biosynthesis of the oil. By desaturating stearic acid (C18:0), it has been proposed that this enzyme is responsible for the accumulation of oleic acid (C18:1) (Mckeon and Stumpf 1982). The second desaturation of oleic acid in linoleic acid is catalyzed by ∆-12 desaturase, another enzyme that contributes to the formation of oleic acid (Stymne and Appelqvist 1980). Palmitic (C16:0) and stearic acid (C18:0) saturated fatty acids were increased significantly in response to water stress. This indicates that palmitic acid and stearic acid may play an important role in drought tolerance mechanism of plants (Hashem et al. 2011).
Nevertheless, treatment of sunflower seedlings with Sts could lessen the impact of drought stress on growth, RWC%, and yield related traits. The obtained data are comparable to those obtained in flax and wheat plants by Hassan et al. (2021) and Hussein et al. (2022). Concurrently, Sts play a regulatory role in plant development (He et al. 2003). Among these functions are cell expansion and vascular differentiation (Rao et al. 2002). Hartmann (1998) stated that phytosterols are biogenic precursors for many components important for plant growth, including membrane permeability and modulation of enzyme activity. Additionally, Rogowska and Szakiel (2020) stated that these improvements might be due to improving water uptake and utilization efficiency, improving cell division, and/or cell enlargement. Moreover, Sts foliar spraying improved the impaired effects of water stress on photosynthetic pigment components. These findings reinforce earlier research in which exogenously applied Sts increased photosynthetic pigment levels in chickpea and Arabidopsis under salt and drought stress (Li et al. 2012). Refereeing to the earlier investigations BRs caused increases in different photosynthetic pigments of wheat (Dong et al. 2017), flax (Bakry et al. (2019), and duckweed (Chmur and Bajguz 2021). This improving effect could be related to its ability to boost oxide assimilation in the Calvin cycle by enhancing RuBisCO’s initial activity. Stigmasterols (Sts) are phytohormones that regulate plant defense responses (Sharma et al. 2014), in addition to enhanced photosystem II efficiency (Filova et al. 2013). Chmur and Bajguz (2021) reflected those increases in pigment contents to the improvement of plant ability to absorb light and the stimulation of the activity of enzymes involved in chlorophyll production. Additionally, the increases in chlorophyll a/b ratios resulted by stigmasterol treatment may be caused via more rapid degradation of chlorophyll b than chlorophyll a. This may be clarified by the fact that the first step in the degradation of chlorophyll b includes conversion to chlorophyll a despite that indole acetic acid sustains the protective role of many crops against biotic and abiotic stress through antagonistic or synergistic interaction with other phytohormones such as gibberellic acid, cytokinin, and abscisic acid. The enhanced IAA levels obtained in response to stigmasterol treatments may lead to an enhancement of enzyme activity and as a result, increased growth parameters in stigmasterol-treated plants (Bakry et al. 2019). Hussein et al. (2022) stated that stigmasterol treatment improved numerous endogenous phytohormones (including IAA) which play an important role in plant development as signals and regulators. Exogenous Sts also improved membrane leakage and at the same time decreased lipid peroxidation and lipoxygenase activity by lowering ROS levels. Lowered ROS production maintains the structural and functional integrity of membranes, maintaining cellular function (Nahar et al. 2016). The positive impact of Sts in defending membranes and stabilizing the key cellular processes is demonstrated by the decreased ROS overproduction and lipoxygenase activity in seedling treated with Sts. Recent research on soybean plant showed that Sts application lowered lipoxygenase activity and ROS production, which led to a decline in lipid peroxidation under water stress (Hasan et al. 2020).
Similar results are obtained earlier by Bassuany et al. (2014) on flax plants. The possible mechanism for improved membrane leakage in response to stigmasterol treatment was the obtained decrease in lipid peroxidation (as indicated by MDA content) in Sts-treated plants. Furthermore, the induction of SOD upregulation, which has aided in the regulation of photosynthetic activity and the structural and functional maintenance of PSII, may be the cause of STs’ treatment effects on antioxidant enzymes in sunflower plants. According to Hassan et al. (2021), stigmasterol treatment resulted in enhanced activity and transcript levels of certain antioxidant enzymes, which improve drought tolerance. Additionally, plants treated with Sts showed an increase in non-enzymatic antioxidants; at the same time, H2O2 and MDA levels dropped, proving that Sts had repaired the antioxidant systems. Sts may therefore lead to an enhancement in the antioxidant system, enhancing ROS scavenging and resulting in increased drought tolerance (Hassan et al. 2021). Improved DPPH%, GSH and α-toco accumulation due to Sts treatment might have significantly contributed to drought tolerance through (a) stabilizing redox homeostasis, (b) scavenging ROS, and (c) preserving the activity of ascorbate-glutathione cycle enzymes including APX and GR. Additionally, GSH plays a crucial signaling role in shielding photosynthetic pigments from stress brought on by oxidative conditions (Foyer and Shigeoka 2011). Increased GSH production in conjunction with GR upregulation is important for maintaining NADP levels for steady photosynthetic electron transport. The same results were obtained in soybean plants under oxidative stress (Hasan et al. 2020). Sts improved yield as well as growth, ROS homeostasis, TPh, antioxidant enzymes, antioxidant, and Rubisco as found by Bassuany et al. (2014) and Hassan et al. (2021).
Stigmasterol treatments markedly enhanced sunflower seed output and its constituents, including oil, total carbohydrate, protein, and flavonoid concentrations, under both normal and drought-stress conditions. Additionally, these increments in carbohydrate levels may be attributed to improved photosynthetic activity, which would accelerate the transfer of carbohydrates from leaves to growing grains (Bassuany et al. 2014). Furthermore, Sts application increased antioxidant activity comparing with control plants. The increase in scavenging activity can be considered an advantage of treatment used that could be attributed to the increases in total phenols and total flavonoids (Yu et al. 2002). The obtained results of increased unsaturated fatty acid content as well as TU/TS (Table 7) in response to Sts treatments might be explained by cell defense against oxidative stress via suppression of lipid peroxidation and free radical scavenging (Velikova et al. 2000). However, the quantity and quality of oil output were greatly enhanced by treatment with Sts. Generally, the concentration of essential fatty acid of an oil determines its quality (Johnson and Bradford 2014), but certain oil quality features suitable for certain industrial uses can also be obtained by water stress.
5 Conclusion
According to our study’s findings, drought stress (50% of water irrigation requirement) reduced sunflower growth attributes, photosynthetic pigments, indole acetic acid, and phenolic. Meanwhile, it increased antioxidant enzymes and antioxidant compounds as well as some osmo-protectants. Furthermore, yield analysis revealed declines in several yield attributes, yielded oil, carbohydrates, and protein percentages, while it increased flavonoid and antioxidant activity percentages as well as altered fatty acid components. These findings supported the negative impact of drought via demonstrating how it stresses sunflower plants. In contrary, exogenous stigmasterol treatment of sunflower plant reduced these negative effects and enhanced quantity and quality of the yielded seeds and oil output. Stigmasterol modulated photosynthetic pigments, ameliorated oxidative stress disorders, accumulated osmo-protectants, enhanced endogenous indole acetic acid contents and phenolic biosynthesis, and triggered superoxide radical- and hydrogen-peroxide-scavenging enzymes. All of these alterations had a positive correlation with the growth, and hence the yield of drought stressed sunflower plants. These were concomitant with improved oil, carbohydrate, protein, and flavonoid contents of seeds as well as fatty acid composition of oil.
References
Abd Elhamid EMA, Sadak MS, Ezzo MI, Abdalla AM (2021) Impact of glycine betaine on drought tolerance of Moringa oleifera plant grown under sandy soil. Asian J Plant Sci 20:578–589. https://doi.org/10.3923/ajps.2021.578.589
Adeleke BS, Babalola OO (2020) Oilseed crop sunflower (Helianthus annuus) as a source of food: nutritional and health benefits. Food Sci Nutr 8:4666–4684. https://doi.org/10.1002/fsn3.1783
Ahanger MA, Ashraf M, Bajguz A, Ahmad P (2018) Brassinosteroids regulate growth in plants under stressful environments and crosstalk with other potential phytohormones. J Plant Growth Regul 37:1007–1024. https://doi.org/10.1007/s00344-018-9855-2
Albalasmeh AA, Berhe AA, Ghezzehei TA (2013) A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr Polym 97:253–261. https://doi.org/10.1016/j.carbpol.2013.04.072
Alqurainy F (2007) Responses of bean and pea to vitamin C under salinity stress. Res J Agric Biol Sci 3:714–722 Corpus ID: 36342705
Angelika F, Sytar O, Rivosudska K (2013) Effects of brassinosteroid on the induction of physiological changes in Helianthus annuus L. under copper stress. Acta Univ Agric et Silvic Mendelianae Brun 3:623–629. https://doi.org/10.11118/actaun201361030623
Anjum F, Yaseen M, Rasul E, Wahid A, Anjum S (2003) Water stress in barley (Hordeum vulgare L.) Effect on chemical composition and chlorophyll contents. Pak J Agric Sci 40:45–49
Aswaq Financial Co. Analysis report of (2018) on the strategic commodities market in Egypt; (24/1/2019). Provided by Engineer Atteia Shaaban, Consultant for Extraction & Refining of Edible Oils; Vice-President of Oils & Oils By-Products Division; Chamber of Food Industry; Federation of Egyptian Industry.
Avramova V, Abdelgawad H, Zhang Z, Fotschki B, Casadevall R, Vergauwen L, Knapen D, Taleisnik E, Guisez Y, Asard H, Beemster GTS (2015) Drought induces distinct growth response, protection, and recovery mechanisms in the maize leaf growth zone. Plant Physiol 169:1382–1396. https://doi.org/10.1104/pp.15.00276
Bakry AB, Sadak MS, Younis ASM (2019) Some agro-physiological studies of stigmasterol on growth and productivity of some flax cultivars under sandy soil conditions. Am Euras J Agron 12:50–63. https://doi.org/10.5829/idosi.aeja.2019.50.63
Bajguz A (2019) Brassinosteroids in microalgae: application for growth improvement and protection against abiotic stresses. In: Hayat S, Yusuf M, Bhardwaj R, Bajguz A (eds) Brassinosteroids: plant growth and development. Singapore, Springer, pp 45–58
Bakhoum GS, Sh SM, Tawfik MM (2022) Chitosan and chitosan nanoparticle effect on growth, productivity and some biochemical aspects of Lupinus termis L. plant under drought conditions. Egypt J Chem 5:537–549. https://doi.org/10.21608/ejchem.2021.97832.4563
Baldini M, Givanardi R, Enferadi ST, Vanozzi GP (2002) Effects of water regime on fatty acid accumulation and final fatty acid composition in the oil of standard and high oleic sunflower hybrids. Ital J Agron 6:119–126
Bano A, Yasmeen S (2010) Role of phytohormones under induced drought stress in wheat. Pak J Bot 42:2579–2587
Bano H, Athar HR, Zafar Z, Kalaji HM, Ashraf M (2021) Linking changes in chlorophyll a fluorescence with drought stress susceptibility in mung bean [Vigna radiata (L.) Wilczek]. Physiol Plant 172:1240–1250. https://doi.org/10.1111/ppl.13327
Bassuany FM, Hassanein RA, Baraka DM, Khalil RR (2014) Role of stigmasterol treatment in alleviating the adverse effects of salt stress in flax plant. Int J Agric Technol 10:1001–1020
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defense and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11:705–719. https://doi.org/10.1111/j.1364-3703.2010.00625.x
Borella J, Becker R, Lima MC, de Oliveira DS, Braga EJ, de Oliveira AC, do Amarante L (2019) Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-633 oxygenation. Sci Agric 76: 51- 62. https://doi.org/10.1590/1678-992x-2017-0195.
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182. https://doi.org/10.38212/2224-6614.2748
Chen G, Asada K (1992) Inactivation of ascorbate peroxidase by thoils requires hydrogen peroxide. Plant Cell Physiol 33:117–123
Chen JX, Wang XF (2006) Plant physiology experimental guide. High Educ Press Beijing, China 24-25:55–56
Chmur M, Bajguz A (2021) Brassinolide enhances the level of brassinosteroids, protein, pigments, and monosaccharides in Wolffia arrhiza treated with brassinazole. Plants 10:1311. https://doi.org/10.3390/plants10071311
Das M, Das SK, Suthar SH (2002) Composition of seed and characteristics of oil from Karingda. Int J Food Sci Technol 37:893–896. https://doi.org/10.1046/j.1365-2621.2002.00638.x
Doderer A, Kokkelink I, Veen VDS, Valk B, Schram A, Douma A (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. BiochimBiophys Acta 1120:97–104. https://doi.org/10.1016/0167-4838(92)90429-H
Dong YJ, Wang WW, Hu GQ, Chen WF, Zhuge YP, Wang ZL, He MR (2017) Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J Soil Sci Plant Nutr 17:554–569 [CrossRef]
El-Hamidi M, Zaher FA, Shaaban A (2020) Edible oil production in Egypt: an overview. Curr Sci Int 9:649–655. https://doi.org/10.36632/csi/2020.9.4.58
Elewa TAE, Sadak MS, Dawood MG (2017) Improving drought tolerance of quinoa plant by foliar treatment of trehalose. Agric Eng Int: CIGR J. 19:245–254
Elkeilsh A, Awad YM, Soliman MH, Abu-Elsaoud A, Abdelhamid MT, El-Metwally IM (2019) Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J Plant Res 132:881–901. https://doi.org/10.1007/s10265-019-01143-5
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA (2019) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem 137:144–153. https://doi.org/10.1016/j.plaphy.2019.02.004
Ezzo MI, Abd Elhamid EM, Sadak MS, Aboelfetoh MA (2018) Improving drought tolerance of Moringa plants by using trehalose foliar treatments. Biosci Res 15:4203–4214
FAO STAT (2022) FAOSTAT database. Food Agric Organ UN Available online
Filova A, Oksana S, Eleonora K (2013) Effects of brassinosteroid on the induction of physiological changes in Helianthus annuus L. under copper stress. Acta Univ Agric Silvic Mendelianae Brun 61:623–629. https://doi.org/10.11118/actaun2013610306232013
Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jounanin L (1995) Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol 109:1047–1057. https://doi.org/10.1104/pp.109.3.1047
Foyer CH, Valadier MH, Migge A, Becker TH (1998) Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiol 117:283–292. https://doi.org/10.1104/pp.117.1.283
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100. https://doi.org/10.1104/pp.110.166181
Geetha S, Sai-Ram M, Mongia SS, Singh V, Ilavazhagan G, Sawhney RC (2003) Evaluation of antioxidant activity of leaf extract of sea buckthorn (Hippo phaerhamnoides L.) on chromium (VI) induced oxidative stress in albino rats. J Ethnopharmacol. 87:247–251. https://doi.org/10.1016/s0378-8741(03)00154-5
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gonza'lez M, Guzma'n B, Rudyk R, Romano E, Molina MAA (2003) Spectrophotometric determination of phenolic compounds in propolis. Am J Pharm 22:243–248
Gruszka D (2013) The brassinosteroid signaling pathway-new key players and interconnections with other signaling networks crucial for plant development and stress tolerance. Int J Mol Sci 14:8740–8774. https://doi.org/10.3390/ijms14058740
Hajlaoui H, Denden M, El-Ayeb N (2009) Changes in fatty acids composition, hydrogen peroxide generation and lipid peroxidation of salt stressed corn (Zea mays L.) roots. Acta Physiol Plant 31:787–796. https://doi.org/10.1007/s11738-009-0293-4
Harbone JP (1984) Phytochemical methods: a guide to modern techniques of plant analysis. Chapman Hall, London
Hartmann MA (1998) Plant sterols and membrane environment. Trends Plant Sci 3:170–175. https://doi.org/10.1016/S1360-1385(98)01233-3
Hasan MM, Ali MA, Soliman MH, Alqarawi AA, Abd-Allah EF, Fang ZW (2020) Insights into 28-homobrassinolide (HBR)-mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J Plant Interact 15:371–385. https://doi.org/10.1080/17429145.2020.1832267
Hasan MM, Alharby HF, Hajar AS, Hakeem KR, Alzahrani Y (2018) Effects of magnetized water on phenolic compounds, lipid peroxidation and antioxidant activity of Moringa species under drought stress. The J Anim Plant Sci 28:803–810
Hashem HA, Bassuony FM, Hassanein RA, Baraka DM, Khalil RR (2011) Stigmasterol seed treatment alleviates the drastic effect of NaCl and improves quality and yield in flax plants. Aust J Crop Sci 5:1858–1867
Hassan NM, Budran IG, El-Bastawisy ZM, El-Harary EH, Nemat AMM (2021) Stigmasterol relieves the negative impact of drought on flax through modulation of redox homeostasis. Egypt J Bot 61:623–635. https://doi.org/10.21608/ejbo.2021.57628.1607
He JX, Fujioka S, Li TC, Kang SG, Setto H, Takatsuto S, Yoshida S, Jang JC (2003) Sterols regulate development and gene expression in Arabidopsis. Plant Physiol. 131:1258–1269. https://doi.org/10.1104/pp.014605
Hodges DM, DeLong JM, Forney C, Prange PK (1999) Improving the thiobarbaturic acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Homme PM, Gonzalez B, Billard J (1992) Carbohydrate content frutane and sucrose enzyme activities in roots, stubble and leaves of rye grass (Lolium perenne L.) as affected by sources/link modification after cutting. J Plant Physiol 140:282–291. https://doi.org/10.1016/S0176-1617(11)81080-1
Huang B, DaCosta M, Jiang Y (2014) Research advances in mechanisms of turfgrass tolerance to abiotic stresses: from physiology to molecular biology. Crit Rev Plant Sci 33:141–189. https://doi.org/10.1080/07352689.2014.870411
Hussein H-AA, Alshammari SO, Elkady FM, Ramadan AA, Kenawy SKM, Abdelkawy AM (2022) Radio-protective effects of stigmasterol on wheat (Triticum aestivum L.) plants. Antioxidants 11:1144. https://doi.org/10.3390/antiox11061144
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354. https://doi.org/10.1016/0304-3770(82)90026-2
Jargar JG, Hattiwale SH, Das S, Dhundasi SA, Das KK (2012) A modified simple method for determination of serum α -tocopherol (vitamin E). J Basic Clin Physiol Pharmacol 223:45–48. https://doi.org/10.1515/jbcpp-2011-0033
Jing M, Shi Z, Zhang M, Zhang M, Wang X (2022) Nitrogen and phosphorus of plants associated with arbuscular and ectomycorrhizas are differentially influenced by drought. Plants 11:2429. https://doi.org/10.3390/plants11182429
Jomo M, Netondo W, Musyimi M (2016) Drought inhibition of chlorophyll content among seven Amaranthuss species. Int J Adv Res Sci Eng Technol 3:1362–1371
Johnson M, Bradford C (2014) Omega-3, omega-6 and omega-9 fatty acids: implications for cardiovascular and other diseases. J glycom lipidom 4:123–131. https://doi.org/10.4172/2153-0637.1000123
Kalsoom U, Bennett IJ, Boyce MC (2016) A review of extraction and analysis: methods for studying osmoregulants in plants. J Chromatogr Sep Tech 7:315. https://doi.org/10.4172/2157-7064.1000315
Kapoor D, Bhardwaj S, Landi M, Sharma A, Ramakrishnan M, Sharma A (2020) The impact of drought in plant metabolism: how to exploit tolerance mechanisms to increase crop production. Appl Sci 10:5692. https://doi.org/10.3390/app10165692
Khan N, Ahmad I, Inamullah M, Muhammad I, Zia U, Ahmad A, Khan D, Khan M, Razzaq A (2016) Morphogenetic screening of Pakistani spring wheat germplasm for drought tolerance. Int J Biosci 8:39–44. https://doi.org/10.12692/ijb/8.5.39-44
Konova IV, Sergeeva YE, Galanina LA, Kochkina GA, Ivanushkina NE, Ozerskaya SM (2009) Lipid synthesis by Geomyces pannorum under the impact of stress factors. Microbiol 78:42–47. https://doi.org/10.1134/S0026261709010068
Kour J, Kohli SK, Khanna K, Bakshi P, Sharma P, Singh AD, Ibrahim M, Devi K, Sharma N, Ohri P, Skalicky M, Brestic M, Bhardwaj R, Landi M, Sharma A (2021) Brassinosteroid signaling, crosstalk and, physiological functions in plants under heavy metal stress. Front. Plant Sci. 12:608061. https://doi.org/10.3389/fpls.2021.608061
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22:289–297. https://doi.org/10.1007/s00344-003-0058-z
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J Exp Biol 20:412–416
Larsen P, Harbo A, Klungron S, Ashein TA (1962) On the biogenesis of some indole compounds in Acetobacter xylinum. Physiol Plant 15:552–565. https://doi.org/10.1111/j.1399-3054.1962.tb08058.x
Li QF, Wang C, Jiang L, Li S, Sun SS, He JX (2012) An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 5:ra72. https://doi.org/10.1126/scisignal.2002908
Li Z, He Y (2020) Roles of brassinosteroids in plant reproduction. Int J Mol Sci 21:872 [CrossRef]
Li S, Zheng H, Lin L, Wang F, Sui N (2020) Roles of brassinosteroids in plant growth and abiotic stress response. Plant Growth Regul 93:29–38 [CrossRef]
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. Curr Protoc Food Anal Chem. https://doi.org/10.1002/0471142913.faf0403s01
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398. https://doi.org/10.1006/anbo.1996.0134
Malkit A, Sadka A, Fisher M, Goldshlag P, Gokhman I, Zamir A (2002) Salt induction of fatty acid elongase and membranes lipid modifications in the extreme halotolerant alga Dunaliella salina. Plant Physiol 129:1320–1329. https://doi.org/10.1104/pp.001909
Marcinska I, Czyczylo-Mysza I, Skrzypek E, Filek M, Grzesiak S, Grzesiak MT, Janowiak F, Hura T, Dziurka M, Dziurka K, Nowakowska A, Quarrie SA (2013) Impact of osmotic stress on physiological and biochemical characteristics in drought- susceptible and drought-resistant wheat genotypes. Acta Physiol Plant 35:451–461. https://doi.org/10.1007/s11738-012-1088-6
Mattioli R, Costantino P, Trovato M (2009) Proline accumulation in plants: not only stress. Plant Signal Behav 4:1016–1018. https://doi.org/10.4161/psb.4.11.9797
Mckeon TA, Stumpf PK (1982) Purification and characterization of the stearol-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem 257:12141–12147. https://doi.org/10.1016/S0021-9258(18)33690-1
Mittler R (2020) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/s1360-1385(02)02312-9
MSTAT-C (1988) A microcomputer program for the design, arrangement, and analysis of agronomic research. Michigan State University, East Lansing
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification system. Ecotoxicol Environ Saf 126:245–255. https://doi.org/10.1016/j.ecoenv.2015.12.026
Nolan TM, Vukašinović N, Liu D, Russinova E, Yin Y (2020) Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32:295–318 [Cross Ref] [Pub Med]
Ozturk M, Unal BT, García-Caparrós P, Khursheed A, Gul A, Hasanuzzaman M (2021) Osmoregulation and its actions during the drought stress in plants. Physiol Plant 172:1321–1335. https://doi.org/10.1111/ppl.13297
Paradiso A, Berardino R, de Pinto MC, di Toppi LS, Storelli MM, Tommasi F, De Gara L (2008) Increase in ascorbate–glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49:362–374. https://doi.org/10.1093/pcp/pcn013
Pathan MS, Subudhi PK, Courtois B, Nguyen HT (2004) Molecular dissection of abiotic stress tolerance in sorghum and rice. In: Nguyen HT, Blum A (eds) Physiology and biotechnology integration for plant breeding. Marcel Dekker, Inc, New York, pp 525–569
Petcu E, Arsintescu A, Stanciu D (2001) The effect of drought stress on fatty acid composition in some Romanian sunflower hybrids. Rom Agric Res 15:39–42
Prabha D, Negi YK (2014) Seed treatment with salicylic acid enhance drought tolerance in Capsicum. World J Agric Res 2:42–46. https://doi.org/10.12691/wjar-2-2-2
Praba ML, Cairns JE, Babu RC, Lafitte HR (2009) Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J Agron Crop Sci 195:30–46. https://doi.org/10.1111/j.1439-037X.2008.00341.x
Ragaey MM, Sadak MS, Dawood MFA, Mousa NHS, Hanafy RS, Latef AAHA (2022) Role of signaling molecules sodium nitroprusside and arginine in alleviating salt-induced oxidative stress in wheat. Plants 11:1786. https://doi.org/10.3390/plants11141786
Rao SSR, Vardhini BV, Sujatha E, Anuradha S (2002) Brassinosteroids-new class of phytohormones. Curr Sci 82(10):1239–1245
Rezazadeh A, Ghasemnezhad A, Barani M, Telmadarrehei T (2012) Effect of salinity on phenolic composition and antioxidant activity of artichoke (Cynara scolymus L.) leaves. Res J Med Plant 6:245–252. https://doi.org/10.3923/rjmp.2012.245.252
Rogowska A, Szakiel A (2020) The role of sterols in plant response to abiotic stress. Phytochem Rev 19:1525–1538 [Cross Ref]
Sadak MS (2022) Nitric oxide and hydrogen peroxide as signaling molecules for better growth and yield of wheat plant exposed to water deficiency. Egypt J Chem (In Press. https://doi.org/10.21608/EJCHEM.2022.117465.5297
Sadak MS, Bakhoum GS (2022) Selenium-induced modulations in growth, productivity and physiochemical responses to water deficiency in Quinoa (Chenopodium quinoa) grown in sandy soil. Biocatal Agric Biotechnol 44:102449. https://doi.org/10.1016/j.bcab.2022.102449
Sadak MS, Ramadan AA (2021) Impact of melatonin and tryptophan on water stress tolerance in white lupine (Lupinus termis L.). Physiol Mol Biol Plants 27:469–481. https://doi.org/10.1007/s12298-021-00958-8
Sadak MS, Bakry BA (2020) Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol Mol Biol Plants 26:907–919. https://doi.org/10.1007/s12298-020-00789-z
Sadak MS, Bakry AB, Taha MH (2019) Physiological role of trehalose on growth, some biochemical aspects and yield of two flax varieties grown under drought stress. Plant Arch 19:215–225
Sardans J, Peñuelas J (2012) The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol 160:1741–1761. https://doi.org/10.1104/pp.112.208785
Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought condition. Plant Cell Environ 25:331–341. https://doi.org/10.1046/j.1365-3040.2002.00754.x
Singh S, Sinha S (2005) Accumulation of metals and its effects in Brassica juncea (L.) Czern. (cv. Rohini) grown on various amendments of tannery waste. Ecotoxicol Environ Saf 62:118–127. https://doi.org/10.1016/j.ecoenv.2004.12.026
Sivakumar P, Sharmila P, Saradhi PP (2000) Proline alleviates salt-stress-induced enhancement in ribulose-1,5-bisphosphate oxygenase activity. Biochem Biophys Res Commun 279:512–515. https://doi.org/10.1006/bbrc.2000.4005
Sharma N, Hundal GS, Sharma I, Bhardwaj R (2014) 28- Homobrassinolide alters protein content and activities of glutathione-s-transferase and polyphenol oxidase in Raphanus sativus L. plants under heavy metal stress. Toxicol Int 21:44–50. https://doi.org/10.4103/0971-6580.128792
Shimoi K, Masuda S, Shen B, Furugori M, Kinae N (1996) Radioprotective effects of antioxidative plant flavonoids in mice. Mutat Res Fund Mol 350:153–161. https://doi.org/10.1016/0027-5107(95)00116-6
Sofy M, Mohamed H, Dawood M, Abu-Elsaoud A, Soliman M (2021) Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch Agron Soil Sci 21:1–22. https://doi.org/10.1080/03650340.2021.1949709
Soltys-Kalina D, Plich J, Strzelczyk-Żyta D, Śliwka J, Marczewski W (2016) The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed Sci 66:328–331. https://doi.org/10.1270/jsbbs.66.328
Sorrequieta A, Ferraro G, Boggio SB, Valle EM (2010) Free amino acid production during tomato fruit ripening: a focus on L-glutamate. Amino Acids 38:1523–1532. https://doi.org/10.1007/s00726-009-0373-1
Sousa CMM, Silva HR, Viera-Jr RGM, Ayres MCC, Costa CLS, Araujo DS, Cavalcante LCD, Barros EDS, Araujo PBM, BrandÃo MS, Chaves MH (2007) Fenóistotais e atividadeantioxidante de cincoplantasmedicinais. Quimica Nova 30(2):351–355. https://doi.org/10.1590/S0100-40422007000200021
Stymne S, Appelqvist LÅ (1980) The biosynthesis of linoleate and α-linolenate in homogenates from developing soya been cotyledons. Plant Sci Lett 17:287–294. https://doi.org/10.1016/0304-4211(80)90159-5
Tariq A, Pan K, Olatunji OA, Graciano C, Li Z, Li N, Song D, Sun F, Wu X, Dakhil MA, Sun X, Zhang L (2019) Impact of phosphorus application on drought resistant responses of Eucalyptus grandis seedlings. Physiol Plant 166:894–908. https://doi.org/10.1111/ppl.12868
Thavaprakash N, Siva-Kumar SD, Raja K, Senthil-Kumar G (2002) Effect of nitrogen and phosphorus levels and ratios on seed yield and nutrient uptake of sunflower hybrid DSH-I. Helia 25:59–68. https://doi.org/10.2298/hel0237059t
Upchurch RG (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 30:967–977. https://doi.org/10.1007/s10529-008-9639-z
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Xiong R, Liu S, Considine MJ, Siddique KHM, Lam HM, Chen Y (2020) Root system architecture, physiological and transcriptional traits of soybean (Glycine max L.) in response to water deficit: a review. Physiol Planta 172:405–418. https://doi.org/10.1111/ppl.13201
Yin TT, Pin UL, Ghazali AHA (2015) Influence of external nitrogen on nitrogenase enzyme activity and auxin production in Herbaspirillum seropedicae (Z78). Trop Life Sci Res 26:101–110
Yu L, Haley S, Perret J, Harris M (2002) Antioxidant properties of hard winter wheat extracts. Food Chem 78:457–461. https://doi.org/10.1016/S0308-8146(02)00156-5
Zhao FG, Qin P (2005) Protective effects of exogenous fatty acids on root tonoplast function against salt stress in barley seedlings. Environ Exp Bot 53:215–223. https://doi.org/10.1016/j.envexpbot.2004.04.001
Zullo MAT, Bajguz A (2019) The Brassinosteroids family—structural diversity of natural compounds and their precursors. In: Hayat S, Yusuf M, Bhardwaj R, Bajguz A (eds) Brassinosteroids: plant growth and development. Springer, Singapore, pp 1–44 [CrossRef]
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hanafy, R.S., Sadak, M.S. Foliar Spray of Stigmasterol Regulates Physiological Processes and Antioxidant Mechanisms to Improve Yield and Quality of Sunflower Under Drought Stress. J Soil Sci Plant Nutr 23, 2433–2450 (2023). https://doi.org/10.1007/s42729-023-01197-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01197-4