Abstract
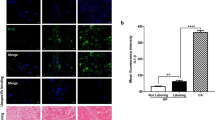
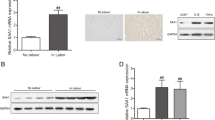
Preterm birth is a major contributor to neonatal deaths and associated long-term morbidities for the survivors, yet therapies remain elusive, given our incomplete understanding of the mechanisms driving human labor and delivery. Human labor is an inflammatory process, and we investigated whether IRG1 (immunoresponsive gene-1) plays a role in these processes. We demonstrate that IRG1 mRNA and protein expression is significantly increased in myometrium with human term labor, compared to no labor samples, and with preterm (LPS) labor in a mouse model. Pro-labor mediators such as pro-inflammatory cytokines TNF and IL1B, and TLR ligands fsl-1, flagellin, LPS, and poly(I:C) also increased IRG1 mRNA expression in myometrial explants. IRG1 silencing, using siRNA in primary myometrial cells, displayed a decrease in the expression of inflammation-induced pro-inflammatory cytokines (IL1A, IL6), chemokines (CCL2, CXCL1, CXCL8), adhesion molecules (ICAM1, VCAM1), and contractility (PTGFR mRNA expression, prostaglandin F2α release, and in situ gel contraction assay). Our results suggest that IRG1 is involved when pro-labor mediators activate the inflammatory processes of human labor, warranting further investigation.






Similar content being viewed by others
References
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72.
Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61.
Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359(3):262–73.
Haas DM, Imperiale TF, Kirkpatrick PR, Klein RW, Zollinger TW, Golichowski AM. Tocolytic therapy: a meta-analysis and decision analysis. Obstet Gynecol. 2009;113(3):585–94.
Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42.
Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200(1):104 e101–11.
Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009;80(1–2):122–31.
Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–5.
Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–36.
Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2):445–9.
Sivarajasingam SP, Imami N, Johnson MR. Myometrial cytokines and their role in the onset of labour. J Endocrinol. 2016;231(3):R101–19.
Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24(1):158–66.
Li Y, Zhang P, Wang C, Han C, Meng J, Liu X, et al. Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species. J Biol Chem. 2013;288(23):16225–34.
Nair S, Huynh JP, Lampropoulou V, Loginicheva E, Esaulova E, Gounder AP, et al. Irg1 expression in myeloid cells prevents immunopathology during M-tuberculosis infection. J Exp Med. 2018;215(4):1035–45.
Hall CJ, Sanderson LE, Lawrence LM, Pool B, van der Kroef M, Ashimbayeva E, et al. Blocking fatty acid-fueled mROS production within macrophages alleviates acute gouty inflammation. J Clin Invest. 2018;128(5):1752–71.
Ren K, Lv Y, Zhuo Y, Chen C, Shi H, Guo L, et al. Suppression of IRG-1 reduces inflammatory cell infiltration and lung injury in respiratory syncytial virus infection by reducing production of reactive oxygen species. J Virol. 2016;90(16):7313–22.
Pan J, Zhao X, Lin C, Xu H, Yin Z, Liu T, et al. Immune responsive gene 1, a novel oncogene, increases the growth and tumorigenicity of glioma. Oncol Rep. 2014;32(5):1957–66.
Papathanassiu AE, Ko JH, Imprialou M, et al. BCAT1 controls metabolic reprogramming in activated human macrophages and is associated with inflammatory diseases. Nat Commun. 2017;8.
Michelucci A, Cordes T, Ghelfi J, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci. 2013;110(19):7820–5.
Lee CGL, Jenkins NA, Gilbert DJ, Copeland NG, Obrien WE. Cloning and analysis of gene-regulation of a novel Lps-inducible Cdna. Immunogenetics. 1995;41(5):263–70.
Hall Christopher J, Boyle Rachel H, Astin Jonathan W, et al. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating β-oxidation-dependent mitochondrial ROS production. Cell Metab. 2013;18(2):265–78.
Hall CJ, Boyle RH, Sun X, et al. Epidermal cells help coordinate leukocyte migration during inflammation through fatty acid-fuelled matrix metalloproteinase production. Nat Commun. 2014;5:3880.
Basler T, Jeckstadt S, Valentin-Weigand P, Goethe R. Mycobacterium paratuberculosis, Mycobacterium smegmatis, and lipopolysaccharide induce different transcriptional and post-transcriptional regulation of the IRG1 gene in murine macrophages. J Leukoc Biol. 2006;79(3):628–38.
Degrandi D, Hoffmann R, Beuter-Gunia C, Pfeffer K. The proinflammatory cytokine-induced IRG1 protein associates with mitochondria. J Interf Cytokine Res. 2009;29(1):55–67.
Tallam A, Perumal TM, Antony PM, Jäger C, Fritz JV, Vallar L, et al. Gene regulatory network inference of immunoresponsive gene 1 (IRG1) identifies interferon regulatory factor 1 (IRF1) as its transcriptional regulator in mammalian macrophages. PLoS One. 2016;11(2):e0149050.
Spach KM, Pedersen LB, Nashold FE, Kayo T, Yandell BS, Prolla TA, et al. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics. 2004;18(2):141–51.
Preusse M, Tantawy MA, Klawonn F, Schughart K, Pessler F. Infection- and procedure-dependent effects on pulmonary gene expression in the early phase of influenza a virus infection in mice. BMC Microbiol. 2013;13:293–3.
Weiss JM, Davies LC, Karwan M, Ileva L, Ozaki MK, Cheng RY, et al. Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors. J Clin Invest. 2018;128(9):3794–805.
Cheon YP, Xu X, Bagchi MK, Bagchi IC. Immune-responsive gene 1 is a novel target of progesterone receptor and plays a critical role during implantation in the mouse. Endocrinology. 2003;144(12):5623–30.
Chen B, Zhang D, Pollard JW. Progesterone regulation of the mammalian ortholog of methylcitrate dehydratase (immune response gene 1) in the uterine epithelium during implantation through the protein kinase C pathway. Mol Endocrinol (Baltimore, Md). 2003;17(11):2340–54.
Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–7.
Lim R, Barker G, Lappas M. A novel role for FOXO3 in human labor: increased expression in laboring myometrium, and regulation of proinflammatory and prolabor mediators in pregnant human myometrial cells. Biol Reprod. 2013;88(6):156.
Lappas M. Expression and regulation of metallothioneins in myometrium and fetal membranes. Am J Reprod Immunol. 2018;80(6):e13040.
Liong S, Lim R, Barker G. Lappas M. Hepatitis A virus cellular receptor 2 (HAVCR2) is decreased with viral infection and regulates pro-labour mediators OA. Am J Reprod Immunol (New York, NY : 1989). 2017;78(1).
Lim R, Barker G, Lappas M. TREM-1 expression is increased in human placentas from severe early-onset preeclamptic pregnancies where it may be involved in syncytialization. Reprod Sci (Thousand Oaks, Calif). 2014;21(5):562–72.
Lappas M. Runt-related transcription factor 1 (RUNX1) deficiency attenuates inflammation-induced pro-inflammatory and pro-labour mediators in myometrium. Mol Cell Endocrinol. 2018.
Lappas M. Runt-related transcription factor 1 (RUNX1) deficiency attenuates inflammation-induced pro-inflammatory and pro-labour mediators in myometrium. Mol Cell Endocrinol. 2018;473:61–71.
Lim R, Barker G, Lappas M. SIRT6 is decreased with preterm labor and regulates key terminal effector pathways of human labor in fetal Membranes1. Biol Reprod. 2013;88(1):17 11-10-17, 11-10.
Lappas M, Permezel M, Georgiou HM, Rice GE. Regulation of phospholipase isozymes by nuclear factor-kappaB in human gestational tissues in vitro. J Clin Endocrinol Metab. 2004;89(5):2365–72.
Lim R, Tran HT, Liong S, Barker G, Lappas M. The transcription factor interferon regulatory Factor-1 (IRF1) plays a key role in the terminal effector pathways of human preterm labor. Biol Reprod. 2015.
Ledingham MA, Thomson AJ, Jordan F, Young A, Crawford M, Norman JE. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet Gynecol. 2001;97(2):235–42.
Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–7.
Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–26.
Tattersall M, Engineer N, Khanjani S, Sooranna SR, Roberts VH, Grigsby PL, et al. Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction. 2008;135(4):569–79.
Singh N, Herbert B, Sooranna GR, Orsi NM, Edey L, Dasgupta T, et al. Is myometrial inflammation a cause or a consequence of term human labour? J Endocrinol. 2017;235(1):69–83.
Lappas M. The IL-1beta signalling pathway and its role in regulating pro-inflammatory and pro-labour mediators in human primary myometrial cells. Reprod Biol. 2017.
Lim R, Barker G, Lappas M. The TLR2 ligand FSL-1 and the TLR5 ligand Flagellin mediate pro-inflammatory and pro-labour response via MyD88/TRAF6/NF-kappaB-dependent signalling. Am J Reprod Immunol (New York, NY : 1989). 2014;71(5):401–17.
Lim R, Barker G, Lappas M. TRADD, TRAF2, RIP1 and TAK1 are required for TNF-alpha-induced pro-labour mediators in human primary myometrial cells. Am J Reprod Immunol (New York, NY : 1989). 2017;78(1).
Lim R, Barker G, Lappas M. TLR2, TLR3 and TLR5 regulation of pro-inflammatory and pro-labour mediators in human primary myometrial cells. J Reprod Immunol. 2017;122:28–36.
Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12(3):145–55.
Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165(4 Pt 1):969–71.
Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578–89.
Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167(4 Pt 1):1041–5.
Nadeau-Vallee M, Chin PY, Belarbi L, et al. Antenatal Suppression of IL-1 Protects against Inflammation-Induced Fetal Injury and Improves Neonatal and Developmental Outcomes in Mice. J Immunol (Baltimore, Md : 1950). 2017;198(5):2047–62.
Cappelletti M, Lawson MJ, Chan CC, Wilburn AN, Divanovic S. Differential outcomes of TLR2 engagement in inflammation-induced preterm birth. J Leukoc Biol. 2018;103(3):535–43.
Ilievski V, Lu SJ, Hirsch E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci (Thousand Oaks, Calif). 2007;14(4):315–20.
Weiner CP, Mason CW, Dong Y, Buhimschi IA, Swaan PW, Buhimschi CS. Human effector/initiator gene sets that regulate myometrial contractility during term and preterm labor. Am J Obstet Gynecol. 2010;202(5):474.e471–20.
Chow L, Lye SJ. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol. 1994;170(3):788–95.
Sparey C, Robson SC, Bailey J, Lyall F, Europe-Finner GN. The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2, and Gs alpha proteins in the upper and lower segments of the human uterus during pregnancy and labor. J Clin Endocrinol Metab. 1999;84(5):1705–10.
Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci U S A. 2010;107(48):20828–33.
Doring B, Shynlova O, Tsui P, et al. Ablation of connexin43 in uterine smooth muscle cells of the mouse causes delayed parturition. J Cell Sci. 2006;119(Pt 9):1715–22.
Sugimoto Y, Yamasaki A, Segi E, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science (New York, NY). 1997;277(5326):681–3.
Brodt-Eppley J, Myatt L. Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstet Gynecol. 1999;93(1):89–93.
Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fat Acids. 1996;54(3):187–91.
Lindberg B. The induction of labour by the intravenous infusion of prostaglandin F2alpha. Prostaglandins. 1977;14(5):993–1004.
Xu C, Liu W, You X, Leimert K, Popowycz K, Fang X, et al. PGF2alpha modulates the output of chemokines and pro-inflammatory cytokines in myometrial cells from term pregnant women through divergent signaling pathways. Mol Hum Reprod. 2015;21(7):603–14.
Lim R, Tran HT, Liong S, Barker G, Lappas M. The transcription factor interferon regulatory factor-1 (IRF1) plays a key role in the terminal effector pathways of human preterm labor. Biol Reprod. 2016;94(2):32.
Khanjani S, Terzidou V, Johnson MR, Bennett PR. NFκB and AP-1 drive human myometrial IL8 expression. Mediat Inflamm. 2012;2012:504952–2.
Van Quickelberghe E, Martens A, Goeminne LJE, Clement L, van Loo G, Gevaert K. Identification of immune-responsive gene 1 (IRG1) as a target of A20. J Proteome Res. 2018;17(6):2182–91.
Lappas M. A20, an essential component of the ubiquitin-editing protein complex, is a negative regulator of inflammation in human myometrium and foetal membranes. Mol Hum Reprod. 2017;23(9):628–45.
Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, et al. Immunoresponsive gene 1 and Itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem. 2016;291(27):14274–84.
ElAzzouny M, Tom CTMB, Evans CR, Olson LL, Tanga MJ, Gallagher KA, et al. Dimethyl Itaconate is not metabolized into Itaconate Intracellularly. J Biol Chem. 2017;292(12):4766–9.
Acknowledgments
The following are gratefully acknowledged: Clinical Research Midwives Genevieve Christophers, Gabrielle Pell, and Rachel Murdoch for sample collection; and the Obstetrics and Midwifery staff of the Mercy Hospital for Women for their cooperation.
Funding
Associate Professor Martha Lappas is supported by a Research Fellowship from the Department of Obstetrics and Gynaecology (University of Melbourne) and a Faculty Fellowship from the University of Melbourne. Funding for this study is provided by the NHMRC (grant no. 1058786), Norman Beischer Medical Research Foundation, the University of Melbourne, and the Mercy Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, R., Lappas, M. Role of IRG1 in Regulating Pro-inflammatory and Pro-labor Mediators in Human Myometrium. Reprod. Sci. 27, 61–74 (2020). https://doi.org/10.1007/s43032-019-00133-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00133-1