Abstract
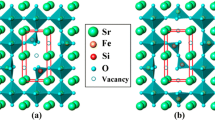
In this study, the mass and charge transport properties of La0.2Sr0.8FeO3-δ have been investigated by deploying defect chemical analysis. From the thermo-gravimetric analysis and DC 4-probe method, oxygen non-stoichiometry (δ) and total electrical conductivity (σ) were examined as functions of temperature (750 < T/°C < 900) and partial pressure of oxygen (10–20 < Po2 /atm < 0.21). The standard enthalpy and entropy changes of reactions and the defect concentrations were calculated from the defect chemical relation. Relative partial molar quantities of mixing of component oxygen were also calculated from both Gibbs–Helmholtz relation and the statistical thermodynamic model. The semiconductor-like conducting behavior due to the localized electrons/holes was observed as with the variation of δ. Electron/hole mobility and oxygen ion partial conductivity were also quantitatively extracted based on the defect structure. Furthermore, first-principles density functional theory (DFT) calculations predict oxidation in Po2 regime Po2 > 10–15–10–12 atm (750–900 °C) can release extra holes and eventually increase electrical conductivity.









Similar content being viewed by others
References
Y.S. Yoo, Y. Namgung, A. Bhardwaj, S.J. Song, A facile combustion synthesis route for performance enhancement of La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF6428) as a robust cathode material for IT-SOFC. J. Korean Ceram. Soc. 56, 497–505 (2019). https://doi.org/10.4191/kcers.2019.56.5.05
T. Ishihara, Nanomaterials for advanced electrode of low temperature solid oxide fuel cells (SOFCs). J. Korean Ceram. Soc. 53, 469–477 (2016). https://doi.org/10.4191/kcers.2016.53.5.469
E. Deronzier, T. Chartier, P.M. Geffroy, Symmetric and asymmetric membranes based on La0.5Sr0.5Fe0.7Ga0.3O3-δ perovskite with high oxygen semipermeation flux performances and identification of the rate-determining step of oxygen transport. J. Eur. Ceram. Soc. 41, 6596–6605 (2021). https://doi.org/10.1016/j.jeurceramsoc.2021.05.060
D.K. Lim, H.N. Im, B. Singh, C.J. Park, S.J. Song, Electrochemical hydrogen charge and discharge properties of La 0.1Sr0.9Co1-yFeyO 3-δ (y = 0, 0.2, 1) electrodes in alkaline electrolyte solution. Electrochim. Acta. 102, 393–399 (2013). https://doi.org/10.1016/j.electacta.2013.03.196
P.M. Bulemo, I.D. Kim, Recent advances in ABO3 perovskites: their gas-sensing performance as resistive-type gas sensors. J. Korean Ceram. Soc. 57, 24–39 (2020). https://doi.org/10.1007/s43207-019-00003-1
J. Mizusaki, M. Yoshihiro, S. Yamauchi, K. Fueki, Nonstoichiometry and defect structure of the perovskite-type oxides La1-xSrxFeO3-°. J. Solid State Chem. 58, 257–266 (1985). https://doi.org/10.1016/0022-4596(85)90243-9
H. Bae, I.H. Kim, B. Singh, A. Bhardwaj, S.J. Song, Defect chemistry of highly defective La0.1Sr0.9Co0.8Fe0.2O3−δ by considering oxygen interstitials: Effect of hole degeneracy. Solid State Ionics 347, 3 (2020). https://doi.org/10.1016/j.ssi.2020.115251
K.J. Lee, J.H. Chung, M.J. Lee, H.J. Hwang, Chromium poisoning of neodymium nickelate (Nd2NiO4) cathodes for solid oxide fuel cells. J. Korean Ceram. Soc. 56, 160–166 (2019). https://doi.org/10.4191/kcers.2019.56.2.07
S. Jiang, J. Sunarso, W. Zhou, J. Shen, R. Ran, Z. Shao, Cobalt-free SrNbxFe1−xO3−δ (x = 0.05, 0.1 and 0.2) perovskite cathodes for intermediate temperature solid oxide fuel cells. J. Power Sources. 298, 209–216 (2015). https://doi.org/10.1016/j.jpowsour.2015.08.063
M. Li, M. Zheng, B. Hu, Y. Zhang, C. Xia, Improving electrochemical performance of lanthanum strontium ferrite by decorating instead of doping cobaltite. Electrochim. Acta. 230, 196–203 (2017). https://doi.org/10.1016/j.electacta.2017.02.014
Y. Lu, H. Zhao, K. Li, X. Du, Y. Ma, X. Chang, N. Chen, K. Zheng, K. Świerczek, Effective calcium doping at the B-site of BaFeO3-: δ perovskite: towards low-cost and high-performance oxygen permeation membranes. J. Mater. Chem. A. 5, 7999–8009 (2017). https://doi.org/10.1039/c7ta00907k
J.S. Yoon, Y.J. Choe, H.J. Hwang, Fabrication of LaySr1-yFexTi1-xO 3-based nanocomposite solid oxide fuel cell anodes by infiltration. J. Korean Ceram. Soc. 51, 224–230 (2014). https://doi.org/10.4191/kcers.2014.51.3.224
V.V. Kharton, A.V. Kovalevsky, M.V. Patrakeev, E.V. Tsipis, A.P. Viskup, V.A. Kolotygin, A.A. Yaremchenko, A.L. Shaula, E.A. Kiselev, J.C. Waerenborgh, Oxygen Nonstoichiometry, mixed conductivity, and mössbauer spectra of Ln 0.5 A 0.5 FeO 3−δ (Ln = La−Sm, A = Sr, Ba): effects of cation size. Chem. Mater. 20, 6457–6467 (2008). https://doi.org/10.1021/cm801569j
H. Wang, C. Tablet, A. Feldhoff, J. Caro, A cobalt-free oxygen-permeable membrane based on the perovskite-type oxide Ba0.5Sr0.5Zn0.2Fe0.8O3-δ. Adv. Mater. 17, 1785–1788 (2005). https://doi.org/10.1002/adma.200401608
B. Bulfin, L. Buttsworth, A. Lidor, A. Steinfeld, High-purity nitrogen production from air by pressure swing adsorption combined with SrFeO3 redox chemical looping. Chem. Eng. J. 421, 127734 (2021). https://doi.org/10.1016/j.cej.2020.127734
Y. Zheng, E.J. Marek, S.A. Scott, H2 production from a plasma-assisted chemical looping system from the partial oxidation of CH4 at mild temperatures. Chem. Eng. J. 379, 122197 (2020). https://doi.org/10.1016/j.cej.2019.122197
E.J. Marek, García-Calvo Conde, Effect of catalyst preparation and storage on chemical looping epoxidation of ethylene. Chem. Eng. J. 417, 127981 (2021). https://doi.org/10.1016/j.cej.2020.127981
V. Celorrio, K. Bradley, O.J. Weber, S.R. Hall, D.J. Fermín, Photoelectrochemical properties of LaFeO3 nanoparticles. ChemElectroChem 1, 1667–1671 (2014). https://doi.org/10.1002/celc.201402192
J.D. Nicholas, Hoghlights from the 2013 national science foundation solid oxide fuel cell promise, progress, and priorities (SOFC-PPP) workshop. Electrochem. Soc. Interface. 22(2013), 49–54 (2013). https://doi.org/10.1149/2.F04134if
F.S. Baumann, J. Fleig, G. Cristiani, B. Stuhlhofer, H.-U. Habermeier, J. Maier, Quantitative comparison of mixed conducting SOFC cathode materials by means of thin film model electrodes. J. Electrochem. Soc. 154, B931 (2007). https://doi.org/10.1149/1.2752974
G. Dong, H. Fan, H. Tian, J. Fang, Q. Li, Gas-sensing and electrical properties of perovskite structure p-type barium-substituted bismuth ferrite. RSC Adv. 5, 29618–29623 (2015). https://doi.org/10.1039/c5ra01869b
H. Lu, J. Tong, Z. Deng, Y. Cong, W. Yang, Crystal structure, oxygen permeability and stability of Ba 0.5Sr0.5Co0.8Fe0.1M 0.1O3-δ (M = Fe, Cr, Mn, Zr) oxygen-permeable membranes. Mater. Res. Bull. 41, 683–689 (2006). https://doi.org/10.1016/j.materresbull.2005.10.017
J. Hombo, Y. Matsumoto, T. Kawano, Electrical conductivities of SrFeO3−δ and BaFeO3−δ perovskites. J. Solid State Chem. 84, 138–143 (1990). https://doi.org/10.1016/0022-4596(90)90192-Z
V.V. Vashuk, L.V. Kokhanovskii, I.I. Yushkevich, Electrical conductivity and oxygen nonstoichiometry of SrCo0.25Fe0.75O3. Inorg. Mater. 36, 1239–1245 (2000)
T. Das, J.D. Nicholas, Y. Qi, Long-range charge transfer and oxygen vacancy interactions in strontium ferrite. J. Mater. Chem. A. 5, 4493–4506 (2017). https://doi.org/10.1039/c6ta10357j
Y. Takeda, K. Kanno, T. Takada, O. Yamamoto, M. Takano, N. Nakayama, Y. Bando, Phase relation in the oxygen nonstoichiometric system, SrFeOx (2.5 ≤ x ≤ 3.0). J. Solid State Chem. 63, 237–249 (1986). https://doi.org/10.1016/0022-4596(86)90174-X
J.P. Hodges, S. Short, J.D. Jorgensen, X. Xiong, B. Dabrowski, S.M. Mini, C.W. Kimball, Evolution of oxygen-vacancy ordered crystal structures in the perovskite series Sr(n)Fe(n)O(3n–1) (n = 2, 4, 8, and ∞), and the relationship to electronic and magnetic properties. J. Solid State Chem. 151, 190–209 (2000). https://doi.org/10.1006/jssc.1999.8640
T. Das, J.D. Nicholas, Y. Qi, Composition, crystallography, and oxygen vacancy ordering impacts on the oxygen ion conductivity of lanthanum strontium ferrite. Phys. Chem. Chem. Phys. 22, 9723–9733 (2020). https://doi.org/10.1039/d0cp00206b
T. Das, J.D. Nicholas, Y. Qi, Polaron size and shape effects on oxygen vacancy interactions in lanthanum strontium ferrite. J. Mater. Chem. A. 5, 25031–25043 (2017). https://doi.org/10.1039/c7ta06948k
M.B. Choi, S.Y. Jeon, H.N. Im, S.J. Song, Thermodynamic quantities and oxygen nonstoichiometry of undoped BaTiO 3-δ by thermogravimetric analysis. J. Alloys Compd. 513, 487–494 (2012). https://doi.org/10.1016/j.jallcom.2011.10.096
P.E. Blöchl, Projector augmented-wave method. Phys. Rev. B. 50, 17953–17979 (1994). https://doi.org/10.1103/PhysRevB.50.17953
G. Kresse, J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 54, 11169–11186 (1996). https://doi.org/10.1103/PhysRevB.54.11169
S. Dudarev, G. Botton, Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B Condens. Matter Mater. Phys. 57, 1505–1509 (1998). https://doi.org/10.1103/PhysRevB.57.1505
Z. Yang, Z. Huang, L. Ye, X. Xie, Influence of parameters u and j in the lsda+u method on electronic structure of the perovskites (formula presented). Phys Rev. B Condens. Matter Mater. Phys. 60, 15674–15682 (1999). https://doi.org/10.1103/PhysRevB.60.15674
I.R. Shein, K.I. Shein, V.L. Kozhevnikov, A.L. Ivanovskiǐ, Band structure and the magnetic and elastic properties of SrFeO3 and LaFeO3 perovskites. Phys. Solid State. 47, 2082–2088 (2005). https://doi.org/10.1134/1.2131149
A.M. Ritzmann, A.B. Muñoz-García, M. Pavone, J.A. Keith, E.A. Carter, Ab Initio DFT+U analysis of oxygen vacancy formation and migration in La1-xSrxFeO3-δ (x = 0, 0.25, 0.50). Chem. Mater. 25, 3011–3019 (2013). https://doi.org/10.1021/cm401052w
M. Kuhn, S. Hashimoto, K. Sato, K. Yashiro, J. Mizusaki, Oxygen nonstoichiometry, thermo-chemical stability and lattice expansion of La0.6Sr0.4FeO3-δ. Solid State Ionics 195, 7–15 (2011). https://doi.org/10.1016/j.ssi.2011.05.013
Ø.F. Lohne, T.N. Phung, T. Grande, H.J.M. Bouwmeester, P.V. Hendriksen, M. Søgaard, K. Wiik, Oxygen non-stoichiometry and electrical conductivity of La 02 Sr 0.8 Fe 0.8 B 0.2 O 3 − δ, B = Fe, Ti, Ta. J. Electrochem. Soc. 161, F176–F184 (2014). https://doi.org/10.1149/2.001403jes
O.V. Merkulov, R.R. Samigullin, A.A. Markov, I.A. Leonidov, M.V. Patrakeev, Defect chemistry and high-temperature transport in SrFe1−xSnxO3–δ. J. Solid State Chem. 243, 190–197 (2016). https://doi.org/10.1016/j.jssc.2016.08.023
J. Yoo, C.Y. Yoo, J.H. Yu, A.J. Jacobson, Determination of oxygen nonstoichiometry in SrFeO3−δ by solid-state coulometric titration. J. Am. Ceram. Soc. 100, 2690–2699 (2017). https://doi.org/10.1111/jace.14755
H. Bae, B. Singh, I.-H. Kim, H.-N. Im, S.-J. Song, Thermodynamic quantities and defect chemical properties of La 0.8 Sr 0.2 FeO 3-δ. J. Electrochem. Soc. 165, F641–F651 (2018). https://doi.org/10.1149/2.0771809jes
H. Bae, J. Hong, B. Singh, A.K. Srivastava, J.H. Joo, S.-J. Song, Investigations on defect equilibrium, thermodynamic quantities, and transport properties of La 0.5 Sr 0.5 FeO 3-δ. J. Electrochem. Soc. 166, F180–F189 (2019). https://doi.org/10.1149/2.0311904jes
H. Bae, B. Singh, L. Mathur, J.H. Joo, S.-J. Song, Defect structure, transport properties, and chemical expansion in Ba 095 La 005 FeO 3– δ. J. Electrochem. Soc. 168, 034511 (2021). https://doi.org/10.1149/1945-7111/abeaed
H.I. Yoo, H. Schmalzried, H. Martin, J. Janek, Cross effect between electronic and ionic flows in semiconducting transition metal oxides. Zeitschrift Fur Phys Chemie. 168, 129–142 (1990). https://doi.org/10.1524/zpch.1990.168.Part_2.129
H.I. Yoo, C.R. Song, D.K. Lee, BaTiO3-δ: Defect structure, electrical conductivity, chemical diffusivity, thermoelectric power, and oxygen nonstoichiometry. J. Electroceram. 8, 5–36 (2002). https://doi.org/10.1023/A:1015570717935
H.-I. Yoo, Defect structure, nonstoichiometry and nonstoichiometry relaxation of complex oxides. J. Korean Ceram. Soc. 44, 2 (2007)
J. Mizusaki, M. Okayasu, S. Yamauchi, K. Fuekio, Nonstoichiometry and phase relationship of the SFeO2.5-SrFeO3 system at high temperature. J. Solid State Chem. 99, 166–172 (1992)
J. Yoo, C.Y. Park, A.J. Jacobson, Determination of the equilibrium oxygen non-stoichiometry and the electrical conductivity of La0.5Sr0.5FeO3-x. Solid State Ionics 175, 55–58 (2004). https://doi.org/10.1016/j.ssi.2004.09.026
J. Cheng, A. Navrotsky, X.D. Zhou, H.U. Anderson, Thermochemistry of La 1-Sr xFeO 3-δ solid solutions (0.0 ≤ x ≤ 1.0, 0.0 ≤ δ ≤ 0.5). Chem. Mater. 17, 2197–2207 (2005). https://doi.org/10.1021/cm048613o
J. Mizusaki, M. Yoshihiro, S. Yamauchi, K. Fueki, Thermodynamic quantities and defect equilibrium in the perovskite-type oxide solid solution La1-xSrxFeO3-δ. J. Solid State Chem. 67, 1–8 (1987). https://doi.org/10.1016/0022-4596(87)90331-8
Y. Shin, K.Y. Doh, S.H. Kim, J.H. Lee, H. Bae, S.J. Song, D. Lee, Effect of oxygen vacancies on electrical conductivity of La0.5Sr0.5FeO3-: δ from first-principles calculations. J. Mater. Chem. A. 8, 4784–4789 (2020). https://doi.org/10.1039/c9ta12734h
M.V. Patrakeev, J.A. Bahteeva, E.B. Mitberg, I.A. Leonidov, V.L. Kozhevnikov, K.R. Poeppelmeier, Electron/hole and ion transport in La1-xSrxFeO3-δ. J. Solid State Chem. 172, 219–231 (2003). https://doi.org/10.1016/S0022-4596(03)00040-9
M.C. Kim, S. Park, H. Haneda, J. Tanaka, S. Shirasaki, High temperature electrical conductivity of La1-xSrxFeO3−δ (x>0.5). Solid State Ionics 40–41, 239–243 (1990)
M. Søgaard, P. Vang Hendriksen, M. Mogensen, Oxygen nonstoichiometry and transport properties of strontium substituted lanthanum ferrite. J. Solid State Chem. 180, 1489–1503 (2007). https://doi.org/10.1016/j.jssc.2007.02.012
I. Wærnhus, T. Grande, K. Wiik, Surface exchange of oxygen in La1-xSrxFeO3-δ (x = 0, 0.1). Top. Catal. 54, 1009–1015 (2011). https://doi.org/10.1007/s11244-011-9712-z
E.V. Tsipis, M.V. Patrakeev, V.V. Kharton, A.A. Yaremchenko, G.C. Mather, A.L. Shaula, I.A. Leonidov, V.L. Kozhevnikov, J.R. Frade, Transport properties and thermal expansion of Ti-substituted La 1-xSrxFeO3-δ (x = 0.5–0.7). Solid State Sci. 7, 355–365 (2005). https://doi.org/10.1016/j.solidstatesciences.2005.01.001
J. Mizusaki, T. Sasamoto, W.R. Cannon, H.K. Bowen, Electronic conductivity, seebeck coefficient, and defect structure of LaFeO3. J. Am. Ceram. Soc. 65, 363–368 (1982). https://doi.org/10.1111/j.1151-2916.1982.tb10485.x
Acknowledgements
This work was supported by the Technology Development Program to Solve Climate Changes of the National Research Foundation (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2017M1A2A2044927) and Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20213030040110).
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bae, H., Shin, Y., Mathur, L. et al. Defect chemistry of p-type perovskite oxide La0.2Sr0.8FeO3-δ: a combined experimental and computational study. J. Korean Ceram. Soc. 59, 876–888 (2022). https://doi.org/10.1007/s43207-022-00237-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-022-00237-6