Abstract
Salinity stress and the absence of light negatively impact growth and development of the plants. Morpho-physiological and biochemical attributes of maize (Zea mays L.) get severely affected by salt stress and subdue light. Therefore, a pot experiment was conducted under the prevailing environmental conditions of Turbat, Balochistan, to explore etiolation and the de-etiolation response of maize hybrid (SP-17S23) to salinity stress under exogenous application of plant growth regulators (PGRs). Maize seedlings in three sets, i.e., non-etiolated, etiolated, de-etiolated, subjected to salinity stress (120 mM NaCl) after 15 days of seed germination. After a week, the seedlings were sprayed with optimized levels of different PGRs, including thiourea (TU; 10 mM), salicylic acid (SA; 250 µM), and kinetin (KIN; 3 µM). Salinity stress hampered plant growth and affected morpho-physiological attributes. However, PGRs foliar treatment proved effective, thus ameliorating the impact of salinity and etiolation on maize seedlings. Growth attributes (root/shoot length, leaf area, root/shoot fresh and dry weight), photosynthetic pigments (Chl a, b and carotenoids) were significantly enhanced under the foliar treatment of PGRs, especially under TU and KIN treatments. However, the oxidative damage parameters, i.e., malondialdehyde (MDA) and hydrogen peroxide (H2O2), decreased under the treatment of PGRs, thereby protecting seedlings under salinity and etiolated conditions. Overall, PGRs enhanced tolerance potential of plants under salinity stress with the consideration of light variations remain the key concern for developing healthy and vigor seedling strands.
Similar content being viewed by others
1 Introduction
Plants growth and development are directly influenced by light which directly or indirectly affects their growth and development; whereas proper red and blue light is required for plant growth [1]. Phytochrome and cytochrome are photoreceptors that detect lights and regulate physiological responses [2]. Etiolation and de-etiolation are influenced by light which plays an important role in plant growth and development [3]. During etiolation (dark) and de-etiolation (light) processes, plants show numerous alterations and variations in morpho-physiological and biochemical attributes [4]. Many changes occur in plant shift from etiolation to de-etiolation phase, such as stimulation of cotyledon, the greening of leaf, reduction of hypocotyl, opening of apical hook, synthesis of anthocyanin, and formation of chloroplast from etioplastic [5].
Salinity is a major problem worldwide, which affects crop productivity. Salt stress alters the morpho-physiological processes like reduction in gas exchange, change in protein synthesis, alteration in antioxidant activity, and disruption of membrane integrity [6,7,8,9]. Moreover, it affects ions accumulation in the root area, cell turgor pressure, and enzyme activity [9, 10]. Particularly, salt stress affects plant's growth in two phases; first, it decreases the soil water potential, and second, it causes salt injury to leaves. Accumulation of salt in older leaves reduces carbohydrates accumulation, translocation of growth hormones, and eventually death [9, 11, 12].
Plant growth regulators (PGRs) are molecules that regulate plants growth. PGRs are natural and synthetic compounds that play an important role in plant growth and development [13,14,15,16]. PGRs regulate physiological and biochemical processes in plants, such as transduction of signal, water uptake, respiration, transport of ions, gas exchanges, and regulation of proteinase inhibitor genes [10, 16,17,18]. Light and PGRs have an overlapping role; however, both act independently and improve plant growth and development [19]. Growth of etiolated plants can be controlled by the supplementation of various PGRs. Many studies showed that PGRs control the transitional processes between etiolation and de-etiolation [20]. Salt toxicity decreases plant dry biomass and nutrients availability,however, application of PGRs increases dry biomass, nutrients uptake contents, and ameliorates toxicity of sodium chloride in plants [10, 21].
Maize (Zea mays L.) is the most important cereal crop [22, 23], which is considered a staple food for greater than 200 million people, and it can be expected that the global population will be eight billion in 2025 [24,25,26]. Salinity affects the physiological processes of maize plants, such as photosynthesis, respiration, transpiration, water retention, seed dormancy, seed germination, hormonal function, and stomatal process. Moreover, under salt stress, seed germination of maize gets hampered [11, 27, 28]. The present study was performed to explore the possible bio-regulatory role of foliar spray of optimized levels of various PGRs including salicylic acid (SA), thiourea (TU), and kinetin (KIN) in the etiolated and de-etiolated maize seedlings under salinity stress based on an array of growth and physiological attributes.
2 Materials and methods
2.1 Experimental layout
A pot experiment was conducted in the prevailing environmental conditions of Turbat Balochistan, Pakistan, to evaluate the etiolation/de-etiolation response of maize hybrid “SP-17S23” under salinity stress and explore the possible role of exogenously applied PGRs in alleviating salinity in etiolated seedlings (Figure S1). Maize hybrid seeds (SP-17S23) were provided by the NIAB, Faisalabad, Pakistan. Pots of 32 cm diameter were filled with 10 kg of soil. The soil ratio was 70:20:10., i.e., soil, sand, and manure, respectively. Prior to sowing soil was analyzed for its pH, EC (electrical conductivity) and OM (organic matter) (Table. 1). Ten seeds were sown per pot at 1 inch depth. The design was completely randomized design (CRD) under factorial with 3 replicates. Two complete sets, i.e., etiolation (devoid of sunlight) and de-etiolation (etiolated seedlings when shifted to sunlight), were generated in comparison to non-etiolated (normal growing plants under sunlight). Etiolation pots were covered with a black sheet because etiolation seedlings should be devoid of sunlight. Moreover, these three combinations of etiolation were placed in two separate sets, i.e., control (Con = no salinity stress) and salinity stress (SS). Thus, a total of 72 pots were used. After 15 days of seed germination, NaCl (120 mM) was applied as soil medium supplementation to create saline environment. After 1 week of salinity stress, plants were foliarly sprayed with optimized levels of PGRs, i.e., thiourea (TU; 10 mM), salicylic acid (SA; 250 µM) and kinetin (KIN; 3 µM). Concentration levels were selected on preliminary trials (data not shown), and thus one best level was selected for the present study. After 1 week of PGRs treatment, plants were harvested and were taken to the laboratory for various morpho-physiological analysis (Table 2).
2.2 Morphological and physiological determination
Plants were removed from pots carefully and thoroughly washed with tap water. Shoot and root length as noted using a scale in cm (plants were carefully cut to separate and measure the shoot and root length). Shoot and root fresh/dry weight was recorded using electric balance. The number of leaves and leaf area were also noted for each treatment.
2.2.1 Photosynthetic pigments analysis
To measure the photosynthetic pigments, 0.1 g of leaves were taken for analysis that was ground in 1–2 ml of 80% acetone, and final volume was maintained up to 10 mL using 80% acetone. Then the absorbance at 663 and 645 nm were taken against 80% acetone as blank. The chlorophyll contents were estimated using the formula described by Arnon [29].
While carotenoid was analyzed by using the formula described by Kirk and Allen [30].
where A480, A645, and A663 were the absorbances of the acetone extract at 480 nm, 645 nm, and 663 nm, respectively.
\({\text{V}} = {\text{ Volume of acetone extract }}\left( {{\text{mL}}} \right).\)
\({\text{W}} = {\text{ Weight of leaf }}\left( {\text{fresh weight in gram}} \right).\)
2.2.2 Oxidative damage measurement
2.2.2.1 Hydrogen peroxide (H2O2) content
Velikova, Yordanov [31] method was used to determine the H2O2 concentration. Briefly, 0.1 g of fresh shoot and roots were homogenized in 1 mL of 0.1% (W/V) Trichloroacetic acid (TCA) in an ice bath. A 0.5 mL of supernatant was taken and added 0.5 mL of the phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide (KI). Vortexed the mixture, and absorbance was measured at 390 nm. Distilled water was used as blank.
2.2.2.2 Malondialdehyde
Malondialdehyde (MDA) was determined by the Heath [32] method. Briefly, 0.1 g of shoot and roots was ground in 1 mL of (1% w/v) TCA. Then, 1 mL of supernatant was taken and mixed with 1 mL of 0.5% thiobarbituric acid (TBA) in 20% TCA [0.5% in 20% (w/v) TCA] and kept in a water bath preheated at 95 oC for 50 min. This material was cooled in an ice bath. Absorbance was measured at 532 nm and 600 nm, and1% TCA was used as blank.
2.3 Statistical analysis
The data was statistically analyzed using STATISTIX 8.1 software (Tallahassee, FL, USA) for ANOVA of CRD under factorial with three biological replications. Furthermore, LSD (least significant difference) was used and the labels/alphabets were added to graph bars of statistically significant attributes (P < 0.05) The graphs, mean, and standard deviation were calculated using “MS EXCEL”.
3 Result
3.1 Morphological/growth attributes
3.1.1 Shoot length
Data recorded for shoot length (SL) showed statistically significant (P < 0.05) results under salinity stress in response to etiolation and de-etiolation regarding the foliar treatment of different PGRs. The result revealed that shoot length was higher under the foliar spray of TU in response to etiolation condition. In contrast, the lowest shoot length was noted under salinity (etiolation) condition with no foliar spray (NFS) (Fig. 1A). In etiolated seedlings (control conditions/no salinity stress) TU was the most effective treatment, enhanced SL by 148.39%, while under SS KIN treatment proved effective with an increase of about 100% in SL. Moreover, in de-etiolated seedling SA (115.38%) and KIN (12.81%) improved SL in control and SS seedlings, respectively.
Effect of salinity stress on the growth parameters (A = shoot length, B = root length, C = shoot fresh weight, D = shoot dry weight) of maize hybrid under non-etiolated, etiolated, and de-etiolated conditions in response to PGRs. Same letters on graphs represent statistically similar effect (P < 0.05). Note: red highlighted bars are the best foliar treatment under non-etiolated, etiolated, and de-etiolated conditions
Overall, the data revealed that the PGRs enhanced shoot length; and notably, the foliar spray of TU and KIN under salinity condition were most effective, which significantly enhanced the shoot length, thereby ameliorating the negative impacts of salinity (Fig. 1A).
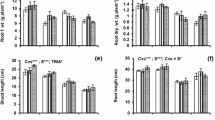
3.1.2 Root length
Data observed for root length (RL) of reported statistically significant (P < 0.05) differences under salinity stress in response to etiolation and de-etiolation and with respect to the foliar treatment of different PGRs. The data further revealed that the highest root length was observed in KIN foliar spray under salinity (De-Etiolation) condition (Fig. 1B). In contrast, the lowest root length was recorded in KIN foliar treatment under etiolation. The result signifies that under un-etiolated seedlings, the maximum length of maize root was observed with SA foliar spray under salinity stress. In the etiolated seedlings, NFS had the longest roots. Hence, under de-etiolation conditions, root length increased with KIN foliar spray under saline conditions while control with minimum root length (Fig. 1B).
In a nutshell, root length under etiolation decreased about 15.38–57.85%. However, under de-etiolation (no salinity stress), RL improved 10.62–33.63% with KIN to be the most effective treatment. KIN further to be the best foliar treatment under de-etiolation (salinity stress).
3.1.3 Shoot fresh weight
Data obtained for shoot fresh weight (SFW) of maize plants under salinity stress by applying PGRs treatments reported statistically non-significant (P > 0.05) results. Data revealed that maximum shoot fresh weight (1.41 g) was observed in non-etiolated seedlings under control while the minimum shoot fresh weight (0.19 g) was observed under etiolation with foliar spray of KIN (Fig. 1C).
In non-etiolated seedlings, SA improved SFW upto 96.79%. However, under salinity stress, a decrease of about 16.27–25% was observed. In etiolated seedlings, TU was the most effective treatment; it increased SF about 86.78% and 118.37% in control and salinity stress, respectively. However, in de-etiolated seedlings, TU (44.80%) and KIN (25.59%) under control and salinity was the most effective treatment to improve SFW, respectively.
3.1.4 Shoot dry weight
Data recorded for shoot dry weight (SDW) of maize grown under salinity stress in response to etiolation de-etiolation by applying PGRs reported statistically non-significant (P > 0.05) results. Data further revealed that in non-etiolated seedlings KIN spray under salinity stress was best treatment that enhanced SDW substantially (0.15 g) while etiolated seedlings under KIN foliar spray and salinity stress had the least SDW (0.33 g) (Fig. 1D).
In etiolated seedlings, TU was the most effective treatment improved SDW by 50%, while salinity decreased SDW by 12.50–37.50%. However, in de-etiolated seedlings KIN (42.31%) and SA (19.23%) was the most effective treatment under control and salinity, respectively.
3.1.5 Root fresh weight
Data recorded for root fresh weight (RFW) of maize grown under salinity in response to etiolation and de-etiolation with PGRs treatments showed statistically significant (P > 0.05) results. Data revealed that maximum fresh weight of root (0.67 g) was reported in non-etiolated seedlings under salinity stress with the foliar application of SA while the minimum RFW (0.06 g) was observed in etiolated seedlings under KIN spray (Fig. 2C). The root fresh weight of seedlings of maize further illustrated that under non-etiolated and de-etiolation conditions, all foliar spray treatments were observed to enhance but particularly the spray of SA under both salinity and control conditions displayed maximum root fresh weight (Fig. 1).
Effect of salinity stress on the growth parameters (A = number of leaves plant−1, B = leaf area, C= root fresh weight, D = root dry weight) of maize hybrid under non-etiolated, etiolated, and de-etiolated conditions in response to PGRs. Same letters on graphs represent statistically similar effect (P < 0.05). Note: red highlighted bars are the best foliar treatment under non-etiolated, etiolated, and de-etiolated conditions.
In a nutshell, the above results revealed that SA foliar spray was the best and most effective treatment as compared to other PGRs.
3.1.6 Root dry weight
Data recorded for root dry weight (RDW) of maize plants grown non-etiolated, etiolated, and de-etiolated seedlings under salinity stress statistically demonstrated non-significant (P > 0.05) results. Results revealed that the maximum root dry weight (0.133 g) was observed with foliar spray of KIN under salinity stress, while in Etiolated and de-etiolated seedlings under salinity and NFS had minimum root dry weights (0.33 g) (Fig. 2D). Further, results indicated that in control etiolated seedlings, NFS was effective treatment. However, in control de-etiolated seedlings, foliar treatment of KIN and TU was quite effective (Fig. 2D).
3.1.7 Number of leaves per plant
Results obtained for number of leaves per plant in maize grown under salinity stress by the application of PGRs showed statistically non-significant (P > 0.05) results. Data further demonstrated that KIN foliar treatment produced plants with maximum leaves under both control and salinity stress in non-etiolated plants. Under etiolation conditions, a greater leaf number was observed with TU spray under stress, and the lowest number of leaves were noted with KIN spray while under de-etiolation conditions, an enhancement was reported in NFS under salinity stress and least number of leaves under TU treatment of salinity stress (Fig. 2A).
Considering overall results for the parameter such as number of leaves counted for maize seedlings reported having increased by applying PGRs and suppressing the effects of salinity in maize seedlings. Particularly KIN, along with TU spray, was observed to increase the number of leaves/plant (Fig. 2A).
Furthermore, in etiolated seedlings under control and salinity stress, a decrease in the number of leaves was observed under all PGRs about 6.67–26.67%, and 7.69–15.28%, respectively. However, de-etiolated seedlings also showed a reduction in the number of leaves under control (8.33–16.67%) and salinity stress (3.57–50%).
3.1.8 Leaf area per plant
Data obtained for leaf area (LA) of Zea mays L. grown under salinity stress in three conditions, i.e., non-etiolated, etiolated, and de-etiolated, statistically showed non-significant (P > 0.05) results. Data revealed that the maximum leaf area was noted in non-etiolated seedlings under control conditions with TU spray. In contrast, the minimum leaf area was observed in etiolated seedlings under control condition with foliar spray of KIN (Fig. 2B).
3.2 Physio-biochemical attributes
3.2.1 Photosynthetic pigments
3.2.1.1 Chlorophyll a
Data obtained for Chl a under salinity stress statistically showed significant (P < 0.05) differences in non-etiolation, etiolation, and de-etiolation conditions under the application of PGRs treatments (Fig. 3 A). The result indicated that the highest Chl a content was noted in non-etiolated seedlings, especially salt-treated. The highest Chl a was noted in salt-treated seedlings with no foliar treatment (NFS), while the minimum was also in NFS but in non-salt stressed seedlings. Etiolated seedlings had the minimum Chl a content as compared to non-etiolated and de-etiolated seedlings, thus providing evidence of the absence of light hampers chlorophyll development. Furthermore, in de- etiolated seedling Chl a content as highest in salt-treated seedlings with KIN foliar treatment and with a minimum in control plants, i.e., without salinity stress (Fig. 3A).
Effect of salinity stress on the photosynthetic pigments (A = chlorophyll a, B = chlorophyll b, C = carotenoids) of maize hybrid under control non-etiolated, etiolated, and de-etiolated conditions in response to PGRs. Same letters on graphs represent a statistically similar effect (P < 0.05). Note: red highlighted bars are the best foliar treatment under non-etiolated, etiolated, and de-etiolated conditions
Etiolation causes a severe reduction in Chl a content in both control and salt-stressed seedlings even under foliar treatment of PGRs. A reduction of about 55.29–81.84% was observed in control seedlings, and 24.29–29.05% decrease was observed in salt-stressed seedlings. However, detiolation causes rapid synthesis of Chl, thereby enhancing Chl a content by 61.05–63.12% and 25.22–54.13% in control and salt-stressed seedlings, respectively. Considering the above results, it can be concluded that under and under etiolation, the biosynthesis of Chl gets hampered, thus manifesting that for healthy seedling growth, light plays a pivotal role (Fig. 3A).
3.2.1.2 Chlorophyll b
Statistical analysis of data obtained for Chl b showed significant (P < 0.05) differences under salinity stress in non-etiolated, etiolated, and de-etiolated seedlings under the exogenous application of different PGRs. Data revealed that Chl b content was higher in non-etiolated seedlings as compared to etiolated and de-etiolated maize seedlings. TU foliar treated seedlings showed the highest Chl b content while the least content was observed in KIN supplied seedlings under salinity stress. Etiolated seedlings foliar treated with SA and KIN showed maximum Chl b content as compared to other PGR treatments in both control and salinity-treated plants. However, in de-etiolated seedlings, maximum Chl b content was recorded with foliar spray of SA under salinity stress, and minimum content was observed under the foliar spray of KIN (Fig. 3B).
In a nutshell, it has been indicated from the results that among all PGRs, TU and SA were highly significant in enhancing the Chl b content in maize seedlings (Fig. 3).
3.2.1.3 Carotenoids
Data recorded for carotenoids of maize seedlings grown under salinity stress in non-etiolated, etiolated, and de-etiolated showed statistically significant (P < 0.05) differences among the application of various PGRs. Data revealed that carotenoid contents enhanced under salinity stress, especially in non-etiolated seedlings. However, de-etiolated seedlings had the least carotenoid contents (Fig. 3C).
Overall results suggest that the carotenoid increased under stress conditions. The photosynthetic pigments are considered to be the main part of plant nourishment, growth, and development. Under saline conditions, foliar spray of PGRs, i.e., TU, KIN, and SA, significantly enhanced carotenoid contents in maize seedlings (Fig. 3).
3.2.1.4 Evaluation of oxidative damage
Hydrogen peroxide (H2O2): Results obtained for H2O2 content in shoot and root showed statistically significant (P < 0.05) results in Zea mays L. in non-etiolation, etiolation, and de-etiolation response, with foliar spray of different PGRs under salinity stress (Fig. 4A, B). Results revealed that foliar spray of KIN under salinity conditions in non-etiolated, etiolated and de-etiolated seedlings, reduced the H2O2 content in the maize shoot (Fig. 4A).
Effect of salinity stress on the oxidative stress parameters (A, B = hydrogen peroxide and C, D = malondialdehyde) of maize hybrid under non-etiolated, etiolated, and de-etiolated conditions in response to PGRs. Same letters on graphs represent statistically similar effect (P < 0.05). Note: red highlighted bars are the best foliar treatment under non-etiolated, etiolated, and de-etiolated conditions
Likewise, data for root H2O2 content also showed significant (P < 0.05) results with foliar spray of different PGRs. Data further revealed that the highest root H2O2 content was noted in etiolated seedlings under control with SA foliar treatment. The lowest root H2O2 content was observed in de-etiolated seedlings with spray of TU under control conditions (Fig. 4B).
Considering etioaled seedlings root and shoot under control conditions, it was observed that the concentration of H2O2 gets elevated 20.21–44.41% (root) and 2.91–13.98% (shoot). However, in salt-stressed seedlings, it increased about 5 folds in both shoot and root. Furthermore, when the seedlings were shifted back to light, i.e., under de-etiolation, a decrease in H2O2 content was observed about 8–27% in root and 5–9% in shoot under PGR application.
Malondialdehyde (MDA): Results obtained for MDA content in shoot and root showed statistically significant (P < 0.05) results in Zea mays L. in non-etiolated, etiolated, and de-etiolated under salinity stress conditions (Fig. 4C, D). Results revealed that NFS under control condition had maximum shoot MDA content (25.48 mmol/g) while spray of SA under salinity of de-etiolation condition had the minimum shoot MDA (8.97) content. In etiolated seedling, reduction of shoot MDA content was observed under KIN treatment under salinity. However, in de-etiolated seedlings, minimum shoot MDA was noticed in SA spray under salinity conditions (Fig. 4C).
Meanwhile, the results obtained for root MDA showed that in non-etiolated seedlings, reduction of root MDA content was noted in TU spray under salinity condition vice versa; an increase was observed with the same foliar treatment, i.e., TU under control conditions. SA foliar treatment in etiolated and de-etiolated seedlings under salinity stress helped to reduce MDA content in root, thus reducing the oxidative stress (Fig. 4D).
Etiolattion enhanced the concentration of MDA in both shoot and root of maize seedlings under control as well as under salt stress. An increase of 33.08% (root) and 4% (shoot) was observed under control. On the other hand, in salt-stressed plants, under etiolation showed an increase of 6.85% root and 3.33% shoot; however, foliar treatment of PGRs tend to restrict the enhancement of MDA in plant tissues.
4 Discussion
Salinity stress is a major abiotic stress that affects plant growth and productivity [9, 33]. It affects plant's morphological, physiological and anatomical attributes. Major impacts include reduction in gas exchange, effect on protein synthesis, altered the activities of antioxidants and damage membrane integrity [6,7,8,9]. Plant growth regulators (PGRs) are molecules that regulate plants growth. PGRs are natural and synthetic compounds that play an important role in plant growth and development [13, 14, 16, 18, 34]. The present research showed that foliar spray of PGRs improved various morphological attributes of maize grown under salinity stress and etiolation phenomenon (Figs. 1, 2). Zahra, Raza [27] reported that Zea mays L. is most susceptible to salt stress than other crops; saline stress reduced the shoot length. However, when plants are transferred from de-etiolation to etiolation, enzymes become active, stem and shoot length get reduced [35].
It has been reported by the study of Taiz, Zeiger [5] that an optimum amount of light is required for increasing biomass, growth, and development for plant. Exogenous application of gibberellin (GA3) or KIN increased fresh and dry matter of shoot of salt-stressed maize, wheat, and cotton plants as compared to control plants. This stimulatory effect was more pronounced at lower and moderate salinization levels. However, spraying with any of the two hormones (GA3 or Kinetin) resulted in more or less comparable values with those of control in the shoot [36]. Our results are in good consideration with the literature that plant growth regulators help plants in enhancing the Morpho-physiological attributes; however, the present study suggests that under saline conditions, KIN foliar spray under non-etiolated conditions enhanced the shoot dry weight (Fig. 1D). However, the described results report a gradual and severe reduction in the shoot dry weight of etiolated seedlings. An important consequence of etiolation is the production of weak seedlings, which means that leaf photosynthesis is severely retarded, since this process requires light to convert the etioplast into chloroplasts and support the seedling growth [37]. High biomass production of a plant is associated with the leaf photosynthetic rate that ultimately depends upon the stomatal conductance and quantity of leaf photosynthetic pigments such as Chl a and b [38]. It was observed that shoot fresh weight was higher with spray of thiourea (Fig. 1C). Stress, although reduced the shoot fresh weight in maize, TU reduced the damaging effect of heat stress and increased shoot fresh weight under both control and stress conditions. The findings of the present study are in agreement with [38] which demonstrate that the maximum shoot fresh weight was noted in non-etiolated seedlings under control and without any foliar spray. Minimum fresh weight was observed under etiolation with spray of KIN. There was observed a reduction in the shoot fresh weight by the spray of KIN in etiolated seedling as compared to non-etiolated and de-etiolated that means that light is required to increase the biomass of plants (Fig. 1C).
Exogenous application of PGRs effectively enhances crop growth and productivity under optimal and sub-optimal conditions [16] with light being the key factor. In the present research, the data suggested maximum root dry weight under salinity with KIN spray, while under etiolated conditions, salinity without a foliar spray of PGRs was reported to minimize the dry weight of root (Fig. 2D). Light and PGRs have an overlapping role; both act independently to affect plants' growth and development [19]. The salt-induced adverse effects on plants are mainly in the form of osmotic stress, production of oxidants, membrane damage, deficiency of essential nutrients, etc. Root dry mass of the maize plants decreased under salinity conditions compared to the control plants. The foliar application of Mannitol or TU under salinity maize plants caused a significant increase in plant dry weight. The effect of Mannitol was slightly higher than the TU in increasing dry weights of the salt-stressed maize plants. However,salinity treatment significantly reduced leaf root dry weight compared to that in the control plants. Foliar application of Mannitol or thiourea significantly improved root dry weight in salt-stressed maize plants [38]. Salt stress significantly reduced root dry weight by 20% and 53%. On the other hand, thiourea significantly improved this attribute by 54% and 64% of root dry weight when treated with (400 μM) thiourea, 27% better under stressed conditions and 18% better than the control condition [39].
Noreen and Ashraf [40] reported that foliar spray of SA decreases the salinity and increases the biomass of plants, ion accumulation, and photosynthesis process. The etiolated plants show long hypocotyls, weak cell walls, and extensive loss of photosynthetic pigments due to these characters etiolated plants having the lowest fresh weight compared to control (non-etiolated) and de-etiolated plants [41]. In comparison, the data reported in this research revealed that under salinity conditions, higher fresh weight was noted with SA application in maize plant by the effective supplementation of SA that enhanced the fresh weight of root while lowest fresh weight was reported under an etiolated condition with KIN spray (Fig. 2C). Sanaullah, Wahid [39] documented that salinity reduces leaf area. In this study, it was reported that TU effectively reduced salinity toxicity in maize seedlings (Fig. 3). According to the present data, light and PGRs were effective in enhancing the said attribute of maize seedling. The leaf area of unsprayed plants, excessively decreased under salinization (Fig. 3B). Exogenous application of gibberellin or Kinetin under saline conditions increased leaf area and reduced effects of salinity stress [36]. Salt stress reduced the growth of salt-sensitive plants such as maize, while the cultivation of salt-resistant maize varieties might help to reduce the yield losses [42].
Light absorption improved the photosynthetic pigment content followed by foliar spray. High salt concentration decreased the chlorophyll formation [11]. In the present study it was observed that maximum Chl a content was under salinity stress with no foliar spray. While minimal Chl a was noted with KIN spray under control (etiolated) condition. Foliar spray of KIN was effective in increasing Chl a content in de-etiolation conditions followed by foliar spray of SA (Fig. 3A). However, the etiolated plants have an elongated and weak stem with light yellow to off white seedling color displaying minimum chlorophyll content [43]. KIN is considered a natural plant growth regulator which enhances Chl a content [44]. It was observed that light is essential for the biosynthesis of Chl a, while the absence of light (etiolation) chloroplast synthesis does not occur from protochlorophyllide, which is present in etiolated seedling [45, 46]. Furthermore, highest Chl b content was noted with TU spray under control condition while minimum Chl b was observed with KIN spray under control (de-etiolation) condition, which illustrates that KIN and TU foliar spray were reported to enhance the photosynthetic pigments such as, i.e., Chl b (Fig. 3B). Etiolation substantially reduced chlorophyll b content (Fig. 3). It was noted that foliar spray of SA in maize plants significantly increased Chl b contents compared to untreated plants [47]. Chl b contents were significantly lower in plants grown under NaCl stress than those in the control plants. However, exogenously applied TU increased Chl content in the salt-stressed plants as compared to those of the salt-stressed plants that were not applied with TU [48]. Additionally, it is indicated that light is an important requirement for the biosynthesis of carotenoids, while in the absence of light, the production of carotenoid hampers [45, 46]. The results of the present study indicated that highest carotenoids content was observed under salinity condition with no spray while the lowest carotenoid was observed under etiolation condition with no spray by showing the foliar spray of PGRs was poorly effective in improving carotenoids pigment under etiolation condition. However, in the de-etiolated seedling, foliar spray of TU under stress enhanced the carotenoid pigment (Fig. 3C). It was reported that salinity reduced the carotenoid content in maize seedlings. At the same time, exogenous application of SA ameliorated the adverse effect of salinity by increasing carotenoid contents as well as Chl a and Chl b [49].
Hydrogen peroxide is an important ROS that regulates various developmental and defensive mechanisms in plants by acting as a secondary messenger in cell signaling [50]. The salinity-induced H2O2 contents increases in plant shoots which resulted in the generation of various reactive oxygen species (ROS) [39]. Enhanced production of ROS, damages the photosystem and biological membrane when produced in the organelles like chloroplast, mitochondria, and peroxisome; salt stress enhances the H2O2 concentration [10]. Studies have shown that an exogenous supply of various stress alleviating agents is beneficial for reducing the stress effects [13]. The data recorded in the present study signify that foliar spray of KIN under salinity conditions reduced the H2O2 content in shoot of maize seedlings. In etiolation and de-etiolation, KIN was more effective and reduced the H2O2 concentration. It was important to note that medium supplementation of Thiourea and Kinetin reduced the production of ROS in both shoot and root (Fig. 4A, B). Kaya, Sonmez [38] reported that Salinity stress resulted in a significant increase in H2O2 accumulation with reference to the control (non-saline) plants. However, exogenously applied Mannitol or Thiourea to the salinized plants reduced the levels of H2O2 compared to the salt-stressed maize plants that received no treatment of mannitol or thiourea. Hydrogen peroxide contents increased by increasing the concentration of salt in all plant organs of maize. Sanaullah, Wahid [39] documented that medium supplementation of thiourea reduced ROS production with elevation in the activity of SOD, followed by CAT and POD. It was concluded that the medium supplementation of thiourea could partially alleviate the salinity-induced production of ROS in maize. In addition, salt-stressed conditions, the MDA content has been reported to increase. MDA is an important indicator of oxidative damage in the plant cell, under salt stress conditions it increases membrane permeability and electrolytic leakage [13]. The present study results revealed that no spray under control conditions increased the shoot MDA content while under salinity foliar spray of SA reduced the MDA content in shoot of maize seedlings. It indicated that all PGRs were effective especially SA and KIN that reduced the MDA content in the maize seedlings. (Fig. 4C, D). It has been reported that salt stress causes enhanced accumulation in root MDA; however,foliar spray of TU decreased root MDA contents [39]. Further, sulfur application lowers the MDA contents at all salinity levels. It can be noted that sulfur is an important constituent of various antioxidants that help in decreasing the toxic effects of salinity on plants. Application of sulfur at 40 mM level significantly lowered the MDA contents in maize seedlings [51] while exogenous use of PGRs were successful in reducing the oxidative damage [10]. Thus, the present study recommends the exogenous supplementation of PGRs to enhance the salinity tolerance potential of maize seedlings especially in response to etiolation and de-etiolation.
5 Conclusion
Subdue light limits seedlings' growth and development, especially under saline soil conditions. Application of PGRs induces salt tolerance in plants by improving morpho-physiological attributes (Fig. 5). Salinity enhanced oxidative stress parameters, i.e., (H2O2 and MDA) in maize seedlings. However, foliar treatment of different PGRs helped ameliorate the harmful impacts of salinity in maize seedlings (Fig. 5). The present study gives insights to unveil and explore the impacts of etiolation in response to various PGRs in plants growing under various environmental stresses especially in medicinal plants with reference to their metabolite profile and its impact on their pharmaceutical properties.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Change history
19 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s44187-022-00034-4
References
Agarwal A, Gupta SD, Barman M, Mitra A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ Exp Botany. 2018;156:170–82.
Griffin JHC, Prado K, Sutton P, Toledo-Ortiz G. Coordinating light responses between the nucleus and the chloroplast, a role for plant cryptochromes and phytochromes. Physiol Plant. 2020;169(4):515–28.
Song M-F, Zhang S, Hou P, Shang H-Z, Gu H-K, Li J-J, Xiao Y, Guo L, Su L, Gao J-W. Ectopic expression of a phytochrome B gene from Chinese cabbage (Brassica rapa L. ssp. pekinensis) in Arabidopsis thaliana promotes seedling de-etiolation, dwarfing in mature plants, and delayed flowering. Plant Mol Biol. 2015;87(6):633–43.
Armarego-Marriott T, Sandoval-Ibañez O, Kowalewska Ł. Beyond the darkness: recent lessons from etiolation and de-etiolation studies. J Exp Bot. 2020;71(4):1215–25.
Taiz L, Zeiger E, Møller IM, Murphy A. Plant physiology and development. USA: Sinauer Associates Incorporated; 2015.
Huang P, He L, Abbas A, Hussain S, Hussain S, Du D, Hafeez MB, Balooch S, Zahra N, Ren X. Seed priming with sorghum water extract improves the performance of camelina (Camelina sativa (L.) crantz) under salt stress. Plants. 2021;10(4):749.
Hafeez MB, Raza A, Zahra N, Shaukat K, Akram MZ, Iqbal S, Basra SMA. Gene regulation in halophytes in conferring salt tolerance. In: Handbook of Bioremediation. Elsevier; 2021. p. 341–70. https://doi.org/10.1016/B978-0-12-819382-2.00022-3.
Saddiq MS, Afzal I, Iqbal S, Hafeez MB, Raza A. Low leaf sodium content improves the grain yield and physiological performance of wheat genotypes in saline-sodic soil. Pesquisa Agropecuária Tropical. 2021;51 https://doi.org/10.1590/1983-40632021v5167663.
Raza A, Tabassum J, Fakhar AZ, Sharif R, Chen H, Zhang C, Ju L, Fotopoulos V, Siddique KH, Singh RK. Smart reprograming of plants against salinity stress using modern biotechnological tools. Crit Rev Biotechnol. 2022;1–28. https://doi.org/10.1080/07388551.2022.2093695.
Zahra N, Wahid A, Hafeez MB, Shaukat K, Shahzad S, Shah T, Alyemeni MN. Plant growth promoters alleviate oxidative damages and improve the growth of milk thistle (Silybum marianum L.) under salinity stress. J Plant Growth Regul. 2021;1–26. https://doi.org/10.1007/s00344-021-10498-w.
Zahra N, Mahmood S, Raza ZA. Salinity stress on various physiological and biochemical attributes of two distinct maize (Zea mays L.) genotypes. J Plant Nutr. 2018;41(11):1368–80.
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S. Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol Biochem. 2020;156:64–77.
Zahra N, Wahid A, Shaukat K, Hafeez MB, Batool A, Hasanuzzaman M. Oxidative stress and tolerance potential of milk thistle ecotypes after supplementation of different plant growth-promoting agents under salinity. Plant Physiol Biochem. 2021;166:53–65.
Hafeez MB, Zahra N, Zahra K, Raza A, Khan A, Shaukat K, Khan S. Brassinosteroids: molecular and physiological responses in plant growth and abiotic stresses. Plant Stress. 2021;100029. https://doi.org/10.1016/j.stress.2021.100029.
Mubarik MS, Khan SH, Sajjad M, Raza A, Hafeez MB, Yasmeen T, Rizwan M, Ali S, Arif MS. A manipulative interplay between positive and negative regulators of phytohormones: a way forward for improving drought tolerance in plants. Physiol Plant. 2021;172:1269–90.
Sabagh AE, Mbarki S, Hossain A, Iqbal M, Islam M, Raza A, Llanes A, Reginato M, Rahman M, Mahboob W, Singhal R, Kumari A. Potential role of plant growth regulators in administering crucial processes against abiotic stresses. Front Agron. 2021;3:648694. https://doi.org/10.3389/fagro.2021.648694.
Raza A, Salehi H, Rahman MA, Zahid Z, Madadkar Haghjou M, Najafi-Kakavand S, Charagh S, Osman HS, Albaqami M, Zhuang Y, Siddique KHM, Zhuang W. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front Plant Sci. 2022;13:961872. https://doi.org/10.3389/fpls.2022.961872.
Raza A, Charagh S, García-Caparrós P, Rahman MA, Ogwugwa VH, Saeed F, Jin W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food. 2022;13(1):196–217. https://doi.org/10.1080/21645698.2022.2106111.
Chory J, Peto C, Feinbaum R, Pratt L, Ausubel F. Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell. 1989;58(5):991–9. https://doi.org/10.1016/0092-8674(89)90950-1.
Vandenbussche F, Verbelen JP, Van Der Straeten D. Of light and length: regulation of hypocotyl growth in Arabidopsis. BioEssays. 2005;27(3):275–84.
Saddiq, MS, Iqbal S, Afzal I, Ibrahim AM, Bakhtavar MA, Hafeez MB, Jahanzaib, Maqbool MM. Mitigation of salinity stress in wheat (Triticum aestivum L.) seedlings through physiological seed enhancements. J Plant Nutr. 2019;42(10):1192–1204.
Anwar Z, Basharat Z, Hafeez MB, Khan S, Zahra N, Rafique Z, Maqsood M. Biofortification of maize with zinc and iron not only enhances crop growth but also improves grain quality. Asian J Agric Biol. 2021. https://doi.org/10.35495/ajab.2021.02.079.
Yu H, Khalid MHB, Lu F, Sun F, Qu J, Liu B, Li W, Fu F. Isolation and identification of a vegetative organ-specific promoter from maize. Physiol Mol Biol Plants. 2019;25(1):277–87.
Nyirenda H, Mwangomba W, Nyirenda EM. Delving into possible missing links for attainment of food security in Central Malawi: farmers’ perceptions and long term dynamics in maize (Zea mays L.) production. Heliyon. 2021;7(5):e07130. https://doi.org/10.1016/j.heliyon.2021.e07130.
Yu HQ, Sun FA, Lu FZ, Feng WQ, Zhang YY, Liu BL, Yan L, Fu FL, Li WC. Positive regulation of phytochrome A on shade avoidance in maize. Pak J Bot. 2018;50:1433–40.
Sun F, Ding L, Feng W, Cao Y, Lu F, Yang Q, Li W, Lu Y, Shabek N, Fu F. Maize transcription factor ZmBES1/BZR1-5 positively regulates kernel size. J Exp Bot. 2021;72(5):1714–26.
Zahra N, Raza ZA, Mahmood S. Effect of salinity stress on various growth and physiological attributes of two contrasting maize genotypes. Brazil Arch Biol Technol. 2020;63:e20200072.
Yu H, Feng W, Sun F, Zhang Y, Qu J, Liu B, Lu F, Yang L, Fu F, Li W. Cloning and characterization of BES1/BZR1 transcription factor genes in maize. Plant Growth Regul. 2018;86(2):235–49.
Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. https://doi.org/10.1104/pp.24.1.1.
Kirk J, Allen R. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965;21(6):523–30.
Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66.
Heath R, Lester P. Photoperoxidation in isolated Chloroplasts of fatty acid peroxidation chlorophyll. Arch Biochem Biophis. 1968;125:189–98.
Saddiq MS, Iqbal S, Hafeez MB, Ibrahim AM, Raza A, Fatima EM, Baloch H, Woodrow P, Ciarmiello LF. Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy. 2021;11(6):1193.
Raza A, Tabassum J, Mubarik M, Anwar S, Zahra N, Sharif Y, Hafeez M, Zhang C, Corpas F, Chen H. Hydrogen sulfide: an emerging component against abiotic stress in plants. Plant Biol. 2022;24:540–58. https://doi.org/10.1111/plb.13368.
García-Martinez JL, Gil J. Light regulation of gibberellin biosynthesis and mode of action. J Plant Growth Regul. 2001;20(4):354–68. https://doi.org/10.1007/s003440010033.
Shaddad M. The exogenous amelioration roles of growth regulators on crop plants grow under different osmotic potential. J Stress Physiol Biochem. 2014;10(1).
Xu X, Chi W, Sun X, Feng P, Guo H, Li J, Lin R, Lu C, Wang H, Leister D. Convergence of light and chloroplast signals for de-etiolation through ABI4–HY5 and COP1. Nature Plants. 2016;2(6):1–7.
Kaya C, Sonmez O, Aydemir S, Ashraf M, Dikilitas M. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J Plant Interact. 2013;8(3):234–41.
Sanaullah T, Wahid A, Sadia B, Hanif A, Maqbool N, Arshad T, Kabir Z. Exogenous application of thiourea ameliorates salt stress effects by alleviation of oxidative damage in hybrid maize. J Agric Sci Technol. 2016;6:220–31.
Noreen S, Ashraf M. Alleviation of adverse effects of salt stress on sunflower (Helianthus annuus L.) by exogenous application of salicylic acid: growth and photosynthesis. Pak J Bot. 2008;40(4):1657–63.
Liu X, Li Y, Zhong S. Interplay between light and plant hormones in the control of Arabidopsis seedling chlorophyll biosynthesis. Front Plant Sci. 2017;8:1433.
Richter JA, Erban A, Kopka J, Zörb C. Metabolic contribution to salt stress in two maize hybrids with contrasting resistance. Plant Sci. 2015;233:107–15.
Lu N, Dai L, Luo Z, Wang S, Wen Y, Duan H, Hou R, Sun Y, Li Y. Characterization of the transcriptome and gene expression of tetraploid black locust cuttings in response to etiolation. Genes. 2017;8(12):345.
Klicova S, Sebanek J, Vlasic T. The effect of cytokinins and other plant hormones on the growth of cotyledonary axilars of flax (Linum usitatissimum), sunflower (Helianthus annuus) and pea (Pisum sativum). Plant Soil Environ. 2004;50(4):182–7.
Solymosi K, Bóka K, Böddi B. Transient etiolation: protochlorophyll (ide) and chlorophyll forms in differentiating plastids of closed and breaking leaf buds of horse chestnut (Aesculus hippocastanum). Tree Physiol. 2006;26(8):1087–96.
Solymosi K, Böddi B. Optical properties of bud scales and protochlorophyll (ide) forms in leaf primordia of closed and opened buds. Tree Physiol. 2006;26(8):1075–85. https://doi.org/10.1093/treephys/26.8.1075.
Amin A, El-Kader AA, Shalaby MA, Gharib FA, Rashad E-SM, Teixeira da Silva JA. Physiological effects of salicylic acid and thiourea on growth and productivity of maize plants in sandy soil. Commun Soil Sci Plant Anal. 2013;44(7):1141–55.
Singh T, Sandhu PS, Chahal GK, Walia SS, Foliar thiourea confers moisture stress tolerance in rainfed maize through elevated antioxidative defence system, osmolyte accumulation and starch synthesis grown under different planting methods. J Plant Growth Regul. 2022;1–19. https://doi.org/10.1007/s00344-021-10540-x.
Tufail A, Arfan M, Gurmani AR, Khan A, Bano A. Salicylic acid induced salinity tolerance in maize (Zea mays). Pak J Bot. 2013;45(S1):75–82.
Zhang Y, Wang Y, Wen W, Shi Z, Gu Q, Ahammed GJ, Cao K, Shah Jahan M, Shu S, Wang J. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy. 2020;17:2876–90.
Riffat A, Sajid M, Ahmad A. Alleviation of adverse effects of salt stress on growth of maize (Zea mays L.) by sulfur supplementation. Pak J Bot. 2020;52(3):763–73. https://doi.org/10.30848/PJB2020-3(38).
Acknowledgements
We are thankful to all members of the Department of Botany, University of Balochistan, Quetta, Pakistan, for providing research environment and support to conduct this study.
Funding
This research received no specific funding.
Author information
Authors and Affiliations
Contributions
KS conceived the idea. G, KS, AS, MN, and GB experimented and analyzed the data. G, KS, AS, MN, GB, NZ, MBH, AS, MN and AR helped in data analysis and wrote the first draft. G, KS, AS, MN, GB, NZ, MBH, AR, and AW wrote, reviewed, and improved the final draft. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to correct the fifth and sixth authors’ names, as well as to replace the word “Asterisk” with “Note” in Figures 1–4 legends.
Supplementary Information
Below is the link to the electronic supplementary material.
44187_2022_27_MOESM1_ESM.tif
Supplementary file1 Figure S1. The experimental layout. Abbreviations: NFS, no foliar spray; SA, salicylic acid; TU, thiourea; KIN, kinetin.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Granaz, Shaukat, K., Baksh, G. et al. Foliar application of thiourea, salicylic acid, and kinetin alleviate salinity stress in maize grown under etiolated and de-etiolated conditions. Discov Food 2, 27 (2022). https://doi.org/10.1007/s44187-022-00027-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-022-00027-3