1. Introduction
Major Depressive Disorder (MDD) is the main contributor to disease burden in developed countries, and the third globally [Reference Murray and Lopez1]. Life expectancy of people with depression is on average 15 years less than the general population’s [Reference Lawrence, Hancock and Kisely2]. This excess of mortality is largely attributed to a deteriorated physical health, including higher prevalence of obesity and increased comorbidity with chronic physical conditions (CPCs) [Reference Ohayon and Schatzberg3].
Several studies have found an association between MDD and CPCs [4–9]. A 16-year prospective study found that the presence of chronic illnesses increased the risk of suffering MDD by 50% [Reference Meng and D’Arcy8]. In Europe, the association between MDD and CPCs was studied in a subsample of the ESEMeD study, consisting of 8796 European community-dwelling adults from six European countries — Belgium, France, Germany, Italy, the Netherlands and Spain—. They found that the presence of CPCs significantly increased the odds of having MDD. Functional disability was proposed as a mediator for the relationship [Reference Stegmann, Ormel and de Graaf10]. Other aspects of physical health are also significantly associated with MDD, including poor general health status, disability, and obesity [Reference Farmer, Korszun and Owen5, Reference Gabilondo, Vilagut, Pinto-Meza, Haro and Alonso7, Reference Stegmann, Ormel and de Graaf10, Reference Anderson, Cohen, Naumova and Jacques11].
Evidence of the association between MDD and physical health is still lacking in some areas, including Spain. Obesity, medication use, and physical conditions such as epilepsy, tinnitus, kidney disease, liver disease, anaemia, or vertigo have not been explored in relation to MDD in the Spanish population. Moreover, there is no evidence about the association between MDD and physical health in Andalusia, the most populous region in Spain.
The aim of this study was to explore whether MDD was associated with physical health in a community-dwelling population of Andalusia, southern Spain. Implications for prevention and treatment are discussed, both at the population and clinical levels.
2. Materials and methods
2.1. Context and design
The PISMA-ep is a cross-sectional study based on a sample of Andalusian community-dwelling adults. The southern-most region of the Iberian Peninsula, Andalusia is the second largest and most populous Spanish autonomous community, with nearly 9 million inhabitants. This study is part of the Integral Mental Health Plan for Andalusia (PISMA), a project undertook by the Andalusian Health Service to improve mental health care planning in the area.
Written informed consent was obtained from all subjects. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Research Ethic Committee of the University of Granada. A detailed description of the methodology is available elsewhere [Reference Cervilla, Ruiz and Rodríguez-Barranco12]. The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines were followed where applicable.
2.2. Sample
We estimated that the sample size needed to calculate a 2% prevalence with ±0.5% precision, confidence intervals of 95% and an effect size of 1.5 was 4518.
The sample was created using different levels of stratification. We ensured there was proportional representation from the eight provinces of Andalusia, and also considered city size, dividing the municipalities into urban, intermediate and rural. For each size, we used a simple random method to select municipalities and street routes within each town.
Inclusion criteria were: being between 18 and 75 years old and having lived in Andalusia for at least a year. Exclusion criteria were: illness that precluded the completion of the interview, not speaking Spanish fluently, suffering from severe cognitive impairment or intellectual disability, and usually residing in an institution.
2.3. Measures
Our main outcome was diagnosis of Major Depression, following DSM-IV/ICD-10 criteria. This diagnosis was obtained using the Spanish version of the Mini International Neuropsychiatric Interview (MINI), a brief diagnostic structured interview that generates Axis I DSM-IV and ICD-10 compatible diagnoses for 16 mental disorders, including MDD [Reference Bobes-García13]. The MINI has shown satisfactory psychometric properties, having good rates of validity and reliability when used on a community-based population [Reference Rossi, Alberio, Porta, Sandri, Tansella and Amaddeo14, Reference Otsubo, Tanaka and Koda15].
Anthropometric measures: We registered the height (m) and weight (kg) of each participant, and calculated their Body Mass Index (BMI) by the formula: weight in kilograms divided by height in square metres (kg/m2). We established four BMI categories, following WHO criteria [16]: Underweight (BMI < 18.5 kg/m2), Normal weight (BMI 18.5–24.99 kg/m2), Overweight (BMI 25.0–29.99 kg/m2) and Obesity (BMI ≥ 30 kg/m2). Hip and waist circumferences (cm) were also measured.
Physical health status: Physical health status over the last four weeks was measured using the physical component summary of the 12-Item Short Form Health Survey (SF-12), Spanish version, where higher scores express better health status [Reference Vilagut, Ferrer, Rajmil, Rebollo, Permanyer-Miralda, Quintana, Santed, Valderas, Ribera and Domingo-Salvany17]. The SF-12 is a subset of the generic SF-36 health survey and has shown a high correspondence with the SF-36 when the sample size is ≥500 [Reference Gandek, Ware and Aaronson18].
Chronic physical conditions: Participants answered a questionnaire on medical problems, consisting on a reference list of 21 medical conditions grouped into 15 categories. This self-report questionnaire is based on the list of CPCs elaborated by the US Department of Health and Human Services, with a few necessary modifications, such as the exclusion of mental disorders from the original list. Different versions of this list have been used health surveys with good results [Reference Goodman, Posner, Huang, Parekh and Koh19, Reference Ward, Schiller and Goodman20]. The conditions listed were: allergies (allergic rhinitis, and other allergies), anaemia, cancer, cardiovascular (CV) diseases (embolism, hypertension, and stroke), chronic pain (arthritis, migraine, and other chronic pain), diabetes mellitus (DM), epilepsy, peptic ulcer, hypercholesterolemia, hypo/hyperthyroidism, kidney disease, liver disease, respiratory diseases (asthma, and chronic bronchitis), tinnitus, and vertigo.
Medication: Currently prescribed medication was assessed through self-report using a reference list of 12 common types of medication. This list included: antibiotics, antiallergic medication, antidepressants, antihypertensives, anxiolytics/hypnotics, contraceptives, gastric medication, insulin/oral hypoglycaemic agents, other cardiovascular drugs different from antihypertensives, painkillers, thyroid hormones, and statins.
Respondents were also administered a standard battery of sociodemographic variables, including sex, age, marital status, employment and educational level. The data collected were matched with the information available in the general census records.
2.4. Procedure
Trained psychologists carried out the recruitment and interviewed the participants face-to-face. There were from 5 to 10 interviewers per province, all of which attended a one-week training course imparted by the main researcher. Quality control of data was performed by a supervisor per province, who doubled-checked that all questionnaires had been adequately completed.
One in every 4 consecutive homes in each street route was visited. If there was no response after two calls, the house was visited twice more at different times of the day. If still there was no answer, the house was substituted with the next one available in the street route. When there was a response, the first person available who fulfilled the inclusion criteria was invited to participate in the study.
At the convenience of the participants, interviews took place at their homes or at their general healthcare centre. Data collection was conducted between 2013 and 2014, and it lasted almost a year. Of the 5496 people approached, 4507 agreed to participate in it and completed the interview, amounting for a response rate of 83.7%.
2.5. Statistical analysis
Using the SPSS version 24.0 software, we calculated current (last two weeks) prevalence of MDD among the participants and used χ2 tests to explore the association between MDD and the correlates. We obtained crude Odds Ratios (ORs) and, using a binary logistic regression, we calculated ORs adjusted by age and sex, and constructed a multivariate model. All tests were two-sided, with a p < 0.05 level of significance and 95% confidence intervals.
3. Results
3.1. Sample description
Our sample is composed of 4507 adults (50.9% female and 49.1% male). Mean age was 42.8 years. Current (last two-weeks) prevalence of MDD was 6.5% (95% CI: 5.7–7.2) in the total sample, and 9.4% among people suffering from any CPC. Lifetime prevalence of any CPC was 48.2% in the total sample, and 69.49% among people currently suffering from MDD. Characteristics of the sample are presented in Table 1. Prevalence of CPCs are presented in Fig. 1 and Supplementary Table 1.
3.2. Association between MDD and CPCs
There was a statistically significant association between MDD and any CPC (OR = 2.60; 95% CI: 2.01–3.35; p < 0.001). This association prevailed after adjusting for age and sex (OR: 2.19; 95% CI: 1.67–2.86; p < 0.001). CPCs with the stronger associations in the bivariate analysis were cancer (adjusted OR = 2.12; 95% CI: 1.30–3.45; p = 0.003), tinnitus (adjusted OR = 4.36; 95% CI: 2.43–7.80; p < 0.001), and peptic ulcer (adjusted OR = 4.40; 95% CI: 2.54–7.60; p < 0.001). General physical health status was inversely associated with MDD (adjusted OR = 0.92; 95% CI: 0.90–0.93; p < 0.001).
Full results are shown in Table 2.
3.3. Anthropometric measures
Increased BMI was positively associated with MDD in women (adjusted OR = 1.08; 95% CI: 1.05–1.11; p < 0.001), but not in men (adjusted OR = 0.99; 95% CI: 0.95–1.05; p = 0.916). A similar result was obtained for obesity (women: adjusted OR = 3.05; 95% CI: 2.05–4.55, p < 0.001; men: adjusted OR = 1.05; 95% CI: 0.59–1.88; p = 0.868) and overweight (women: adjusted OR = 1.56; 95% CI: 1.05–2.31; p = 0.029; men: adjusted OR = 0.68; 95% CI: 0.42–1.10; p = 0.114). Waist and hip circumferences were also significantly associated with MDD in women. In men, only waist circumference showed weak correlation with MDD. Full results are shown in Table 3.
3.4. Medication use
In the total sample, 135 participants (3.0%) were taking antidepressants at the time of the interview, while 407 (9.0%) were taking anxiolytics/hypnotics. 21.4% of depressed participants were receiving antidepressant medication and 37.6% were taking anxiolytic/hypnotic medication. Full results are shown in Table 4.
Taking non-psychopharmacological medication was significantly associated with MDD in the bivariate analysis (adjusted OR = 2.27; 95% CI: 1.79–2.88; p < 0.001) (Table 1). However, in the multivariate model (see Table 5), this association disappeared after controlling for presence of CPCs.
After controlling for age, sex and presence of MDD, antidepressants (OR = 1.98; 95% CI: 1.27–3.09; p = 0.003) and anxiolytic/hypnotic medication (OR = 2.49; 95% CI: 1.76–3.52; p < 0.001) were significantly associated with prevalence of CPCs. Association between these medications and obesity was not significant after controlling for the same factors (antidepressants: OR = 1.07; 95% CI: 0.65–1.77; p = 0.795; anxiolytic/hypnotics: OR = 1.06; 95% CI: 0.78–1.44; p = 0.715).
Regarding the specific conditions, after controlling for age, sex and MDD, both antidepressants and anxiolytic/hypnotic medications were significantly associated with the prevalence of all CPCs except for anaemia, cancer, kidney disease, epilepsy, and liver disease.
3.5. Multivariate association models
Seven variables were associated with MDD in the multivariate regression model: female gender (OR = 1.83; 95% CI: 1.31–2.54; p < 0.001), SF-12-measured physical health status (OR = 0.94; 95% CI: 0.92–0.96; p < 0.001), obesity (OR = 1.67; 95% CI: 1.20–2.31; p = 0.002), peptic ulcer (OR = 2.40; 95% CI: 1.16–4.97; p = 0.018), cancer (OR = 4.12; 95% CI: 1.63–10.37: p = 0.003), vertigo (OR = 2.12; 95% CI: 1.28–3.50; p = 0.004), and tinnitus (OR = 2.62; 95% CI: 1.10–6.39; p = 0.034) (see Table 5).
Table 1 Sociodemographic characteristics of the sample.

BMI = Body Max Index; MDD = Major Depressive Disorder; SE = Standard Error.

Fig 1. Prevalence of chronic physical conditions in participants with and without major depressive disorder.
Table 2 Bivariate associations for Major Depressive Disorders: Chronic Physical Conditions & Use of medication.

CI = Confidence Interval; MDD = Major Depressive Disorder; OR = Odds Ratio; SF-12 = 12-Item Short Form Health Survey.
* Statistically significant at p < 0.05.
As for CPCs, five factors were independently associated with lifetime prevalence of any CPC: age, sex, BMI, MDD, and hypnotic/anxiolytic medication (Supplementary Table 2).
4. Discussion
4.1. Prevalence of MDD
Prevalence of MDD appears to be higher in this area than in other parts of Spain. The ESEMED study, using a sample of 5 non-Andalusian Spanish provinces found a lifetime prevalence of 10.6%, and a prevalence in the last year of 4.0% [Reference Gabilondo, Rojas-Farreras, Vilagut, Haro and Fernández21]. Other studies carried out in Spain with smaller sample sizes found a 12-month prevalence of 6% [Reference Navarro-Mateu, Tormo, Salmerón, Vilagut and Navarro22], and a current prevalence ranging from 1.5% to 1.8% [Reference Ayuso-Mateos23, Reference Calvó-Perxas, Garre-Olmo and Vilalta-Franch24].
Cultural idiosyncrasies of the Andalusian region, along with the consequences of the economic recession, which struck this area with particular strength, have been proposed as an explanation for this higher-than-expected prevalence [Reference Porras-Segovia, Valmisa, Gutiérrez, Ruiz and Rodríguez-Barranco25].
4.2. Chronic physical conditions and MDD
Nearly half of the Andalusian population suffers from some kind of CPC. The already-worrying prevalence of MDD increased among these participants, affecting almost one in ten people. Most of CPCs explored were significantly associated with MDD after adjusting for age and sex. Our results are concordant with previous studies developed in Spain and Europe, which indicate high rates of comorbidity and CPCs [Reference Farmer, Korszun and Owen5–Reference Gabilondo, Vilagut, Pinto-Meza, Haro and Alonso7, Reference Scott, Lim and Al-Hamzawi9].
Table 3 Bivariate associations for Major Depressive Disorder: anthropometric variables.

CI = Confidence Interval; OR = Odds Ratio; SE = Standard Error; BMI = Body Max Index.
* Statistically significant at p < 0.05.
Table 4 Medication use among participants with and without Major Depressive Disorder.

Table 5 Multivariate association model for Major Depressive Disorder.
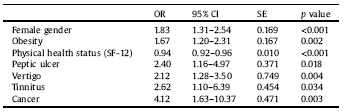
CI = Confidence Interval; OR = Odds Ratio; SE = Standard Error; BMI = Body Max Index; SF-12 = 12-Item Short Form Health Survey.
Results from the World Health Surveys, adding up to a total sample of 245,404 participants from 60 countries, show a significant association between depression and four chronic diseases: angina, arthritis, asthma, and diabetes [Reference Farmer, Korszun and Owen5]. In a 2008 study, several medical disorders, including gastric ulcer, allergic rhinitis, arthritis, thyroid disease, hypertension and asthma, were found to be more prevalent in people with recurrent depressive disorders [Reference Farmer, Korszun and Owen5]. Regarding the Spanish population, a 2012 cross-sectional of study of 2121 community-dwelling adults found that MDD was significantly associated with CPCs and disability [Reference Gabilondo, Vilagut, Pinto-Meza, Haro and Alonso7].
There are several mechanisms by which organic diseases can predispose to a depressive state and viceversa. From a psychological perspective, CPCs can be a traumatic experience that may trigger a depressive episode in predisposed people [Reference Helgeson and Zajdel26]. Suffering from a physical condition can also limit the opportunities to engage in collective activities, thus decreasing social interaction and support, which are vital for psychological well-being. Conversely, people suffering from depression are more likely to have unhealthy lifestyles. They practice less exercise, have an inadequate diet, and substance use is also more prevalent among them than in the general population [Reference Degenhardt, Hall and Lynskey27, Reference Hamalainen28].
Biological factors also play an important role in this connection. Depression has a pro-inflammatory effect, while an increased state of inflammation favours the appearance of depressive symptoms [Reference Miller and Raison29, Reference Stewart, Rand, Muldoon and Kamarck30]. This mechanism is thought to be especially relevant for CV diseases and supports the notion of a two-way relationship between depression and physical conditions [Reference Carney, Freedland, Miller and Jaffe31, Reference Nicholson, Kuper and Hemingway32]. Dysfunction of the hypothalamic-hypophyseal axis has also been proposed as a potential mediator for this association [Reference Farmer, Korszun and Owen5].
Focusing on the association between MDD and specific CPCs, cancer is a well-studied correlate for depression [Reference Burgess, Cornelius, Love, Graham, Richards and Ramirez33–Reference Kim, Kim, Kim, Shin, Bae and Shim36]. The small sample size prevented Gabilondo et al. (2012) from analysing this variable [Reference Gabilondo, Vilagut, Pinto-Meza, Haro and Alonso7]. Consequently, ours is the first Spanish community-based study in finding such association. Awareness of a terminal status in incurable cases is a crucial psychological factor in the triggering of depression [Reference Goodwin, Keyes, Stein and Talley37]. Biological and genetical factors may also be involved in the relationship [Reference Scott, Alonso and de Jonge38, Reference Hsu, Hsu and Chang39].
Some community-based studies have found an association between peptic ulcer and depressive symptoms [Reference Farmer, Korszun and Owen5, Reference Harrop-Griffiths, Katon, Dobie, Sakai and Russo40–Reference McCormack, Edmondson-Jones and Fortnum42]. A retrospective study carried out in 2013 using data from 19 countries, including Spain, found that MDD increased the risk of developing peptic ulcer [Reference Scott, Alonso and de Jonge38]. Nicotine and alcohol dependence, which are more common in depressed people, are thought to play a key role in this relationship [Reference Goodwin, Keyes, Stein and Talley37].
There is limited evidence on the association between MDD and tinnitus. The hearing of a buzzing in the absence of external stimuli is a frequent condition in the general population and is believed to be highly influenced by psychological factors. A few studies in clinical settings have reported an increased risk of MDD among tinnitus patients [Reference Harrop-Griffiths, Katon, Dobie, Sakai and Russo40]. At the population level, an association between tinnitus and nonspecific forms of depression has been reported in a few parts of the world, such as the US or the UK [Reference Shargorodsky, Curhan and Farwell41, Reference McCormack, Edmondson-Jones and Fortnum42].
Vertigo is another condition whose relationship with depression has been scarcely studied before. As with tinnitus, association between MDD and the different types of vertigo has been observed occasionally in clinical settings [Reference Kozak, Dundar and Uca43], but population-based studies report depressive symptoms rather than specific depressive disorders, and no previous evidence is available in the Spanish population. Anxiety derived of the fear of suffering an unexpected episode of vertigo, and restrictions in daily life activity caused by these symptoms, may be some of the factors involved in this association [Reference Kozak, Dundar and Uca43]. Additionally, vertigo is a common side effect of many antidepressants, which may worsen the course of the disorder and increase functional impairment [Reference Kikuchi, Suzuki, Uchida, Watanabe and Mimura44].
4.3. Obesity and other anthropometric variables
Obesity and high BMI have also been associated with MDD in several studies [Reference Stegmann, Ormel and de Graaf10, Reference Tyrrell, Mulugeta and Wood45–Reference Rivera, Locke and Corre48]. In our study, higher BMI as a continuous variable, as well as overweight and obesity, were positively associated with MDD in women, but not in men. Previous studies have reported a similar gender-dependent association, which could be the result of different standards for men and women regarding body weight [Reference Stegmann, Ormel and de Graaf10, Reference Scott, Alonso and de Jonge38]. A 2018 study aimed to explore whether depression was associated with higher BMI, as well as with a genetic risk score (GRS) constructed with 73 obesity-related polymorphisms. Authors found a significant association between depression and both variables, which was also stronger in women than in men [Reference Tyrrell, Mulugeta and Wood45].
The association between BMI and depression is often explained by means of a distortion of body image, stigma associated with obesity, and the resulting low self-esteem [Reference Puhl and Heuer49, Reference Tronieri, Wurst, Pearl and Allison50]. Although the different association between men and women point to a predominance of psychosocial factors behind this relationship, biological factors are also relevant. Obesity and depression may share certain etiopathogenetic aspects, such as shared genetic factors, dysregulation of brain circuits and alterations in the immune-inflammatory system [Reference Milaneschi, Simmons, van Rossum and Penninx51].
4.4. Medication use
Only a quarter of people suffering from Major Depression were receiving antidepressant medication. Although a degree of overdiagnosis of MDD caused by our psychometric tool cannot be ruled out, such a low rate of antidepressant use may be indicating an underdiagnose of MDD in the clinical practice. There is evidence that MDD patients may be undertreated. Data from the WMH surveys from 23 countries, including Spain, show that only 16.5% of people suffering from MDD received minimally adequate treatment for their condition. This figure was 27% in the Spanish population, a similar result to the one obtained in our study [Reference Thornicroft, Chatterji and Evans-Lacko52]. Access to treatment may be difficulted in people suffering from MDD and other mental disorders, partly due to the stigmatization associated with mental health care.
Medication is also a potentially relevant contributor to the relationship between MDD and CPC. A number of non-psychopharmacological treatments have been associated with depression and, inversely, many psychotropics can have detrimental effects on physical health [Reference Udina, Castellví, Moreno-España, Navinés and Valdés53–Reference Martin, Ul-Haq and Nicholl56]. For instance, a cross-sectional study carried out in 145,991 participants found that taking any psychotropic medication significantly increased cardiovascular risk [Reference Martin, Ul-Haq and Nicholl56]. In our study, we did not find non-psychopharmacological medication to be associated with MDD independently of physical conditions. In contrast, use of antidepressants and/or anxiolytic/hypnotic medication significantly increased the prevalence of CPCs after adjusting for potential confounders.
4.5. Implications for clinical practice
Comorbidity goes beyond having two or more conditions at the same time. Diseases interact with each other worsening the patient’s clinical course and exponentially increasing the number of pharmacological interactions, thus limiting the therapeutic options [Reference Martin, Ul-Haq and Nicholl56]. Consequently, collaboration between different departments is essential to guarantee an integral healthcare. Far too often, this is not achieved, especially when mental health comes into play. In addition to the possibility of pharmacological interactions, the treatment of CPCs sometimes requires patients to follow specific lifestyle guidelines or performing self-management behaviors which may be difficult to perform when suffering of MDD [Reference Lin, Katon and Von Korff57, Reference Bane, Hughes and McElnay58]. The frequent stigmatization of the psychiatric patient adds to this clinical challenge, to the extent that psychiatrists are often the main healthcare professional mentally ill patients rely upon [Reference Oosthuizen, Carey and Emsley59]. This situation requires a more holistic approach in the treatment of our patients, both in mental and non-mental health care.
4.6. Strengths & limitations
This is the first study in exploring the association between CPCs and MDD in Andalusia, and the largest one that explores this association in the Spanish population. To our knowledge, the association between MDD and some of the CPCs here considered —including anaemia, epilepsy, kidney disease, liver disease, tinnitus, and vertigo— had not been explored previously in the Spanish population, and a few of them had not been studied in European populations either. This is also the first time that the relationship of MDD with obesity and use of medication is tested in the Spanish population at the epidemiological level.
Our findings need to be considered in light of some limitations. First, the cross-sectional design of the study precludes establishing causality. Associations must be confirmed in longitudinal studies. The necessary selection of chronic physical conditions does not cover the wide range of disorders one can suffer from. Regarding psychopharmacological medication, lack of data on the specific agents used and indication bias precludes a clear interpretation of our results. Finally, a selection bias may exist since patients with mental disorders may be more reluctant to participate.
4.7. Conclusion
In conclusion, we found that MDD was associated with poorer general physical health, obesity, chronic physical conditions, and increased use of medication in the community-dwelling adult population of southern Spain. In women, MDD was also associated with overweight and obesity. The relationship between MDD and CPCs is likely to be bidirectional and of a multifactorial nature, involving several psychological and biological factors that interact with each other. The high rates of comorbidity between MDD and CPC call for a holistic management of physical and mental health in the clinical practice. However, specialization and super-specialization are the tendency in medical sciences due to the rapid increase in knowledge [Reference Oosthuizen, Carey and Emsley59]. An adequate coordination between different departments, with the general practitioner as the central axis, is the best next option.
Role of the funding source
This work was partially supported with a subsidy from the Department of Economy, Innovation and Science of the Regional Government of Andalusia (10-CTS-6682), by the Marie Curie Research Grants Scheme (FP7-626235) and by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation.
Conflict of interest
The authors declare they have no conflict of interest
Acknowledgements
This study was partially financed with a subsidy from the Department of Economy, Innovation and Science of the Regional Government of Andalusia (10-CTS-6682).
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurpsy.2019.04.008.











Comments
No Comments have been published for this article.