Introduction
Cover crops (CC) are widely used for enhancing soil physical, chemical and biological health in agroecosystems (Finney et al., Reference Finney, Buyer and Kaye2017). Worldwide, interest is growing among producers to use CC under different cropping systems for improving soil health and performance (e.g., nutrient cycling (White et al., Reference White, Brennan, Cavigelli and Smith2020), organic matter (Kopittke et al., Reference Kopittke, Dalal, Hoeschen, Li, Menzies and Mueller2020), microbial biomass (Kim et al., Reference Kim, Zabaloy, Guan and Villamil2020; Rankoth et al., Reference Rankoth, Udawatta, Gantzer, Jose, Veum and Dewanto2019a), aggregate stability (Adeli et al., Reference Adeli, Brooks, Read, Shankle, Feng and Jenkins2019; Domagała-Świątkiewicz et al., Reference Domagała-Świątkiewicz, Siwek, Bucki and Rabiasz2019)) and crop production (Austin et al., Reference Austin, Wickings, McDaniel, Robertson and Grandy2017; Ghimire et al., Reference Ghimire, Ghimire, VanLeeuwen and Mesbah2017). However, various CCs under different crop rotations for a range of durations have distinctive effects and functions on soils and microbes (Bacq-Labreuil et al., Reference Bacq-Labreuil, Crawford, Mooney, Neal and Ritz2019; Balota et al., Reference Balota, Calegari, Nakatani and Coyne2014; Fageria et al., Reference Fageria, Baligar and Bailey2005). The legume CCs act as strong N-fixer and convert the N in the atmosphere into the plant available N forms (NH4–N and NO3–N) (Somenahally et al., Reference Somenahally, DuPont, Brady, McLawrence, Northup and Gowda2018). These CCs produce relatively higher N content residue which can be easily broken down and made available for subsequent crops. The grass CCs with well-developed root system has strong nutrient-scavenging ability, especially for N, therefore, has been used to reduce N leaching (Kaspar et al., Reference Kaspar, Jaynes, Parkin, Moorman and Singer2012; Sanchez et al., Reference Sanchez, Fultz, Lofton and Haggard2019a). Biomass from ryegrass CC can enhance soil organic matter and reduce N leaching during winter period and protects the bare soil from wind and water erosion even for the heavy rain during the early spring (Kaye et al., Reference Kaye, Finney, White, Bradley, Schipanski, Alonso-Ayuso, Hunter, Burgess and Mejia2019; Salmerón et al., Reference Salmerón, Isla and Cavero2011). The use of brassicas CC (e.g., radish) with deep root system can supply extra organic matter and nutrients for the following cash crop after the decomposition of roots and reduce the soil compaction (Abdollahi and Munkholm, Reference Abdollahi and Munkholm2014). Therefore, not every single CC can provide all the soil health benefits. Thus, multi-purpose CCs such as integrating legume and grass CC or introducing multispecies mixture CC into the cropping systems can be more beneficial for enhancing the soil health as they can extend the range of substrates to the ecosystem and provide various ecosystem services (Chu et al., Reference Chu, Jagadamma, Walker, Eash, Buschermohle and Duncan2017; Drost et al., Reference Drost, Rutgers, Wouterse, De Boer and Bodelier2020).
CCs increase the diversity of cropping system and microbial activity (Caban et al., Reference Caban, Kuppusamy, Kim, Yoon, Kim and Lee2018; Ding et al., Reference Ding, Liu, Herbert, Novak, Amarasiriwardena and Xing2006). These crops help in improving the soil enzymes such as β-glucosidase and urease (Mukumbareza et al., Reference Mukumbareza, Muchaonyerwa and Chiduza2016), arylamidase (Tang et al., Reference Tang, Xiao, Tang, Lin, Wang and Yang2014), phosphatase (Brooks et al., Reference Brooks, Tewolde, Adeli, Shankle, Way, Smith and Pepper2018; Wei et al., Reference Wei, Guo, Sun, Zhai, Wang, Liu, Hua and Yang2018) and fluorescein diacetate (FDA) (Mendes et al., Reference Mendes, Bandick, Dick and Bottomley1999). Soil microbial biomass, microbial community structure, enzymes and soil protein respond differently to various types of CC (Brennan and Acosta-Martinez, Reference Brennan and Acosta-Martinez2017). Further, these CCs also help in increasing the total phospholipid fatty acid (PLFA), and different CC showed distinctive effect on the proportional distribution of soil microorganisms. For instance, ryegrasses had a more pronounced effect on arbuscular mycorrhizal fungi (AMF) than the legume CC hairy vetch (Finney et al., Reference Finney, Buyer and Kaye2017). Similarly, García-González et al. (Reference García-González, Quemada, Gabriel and Hontoria2016) reported that grass CC barley performed better in enhancing AMF and glomalin-related soil protein (GRSP) content than the legume vetch and no CC (NCC). Chavarría et al. (Reference Chavarría, Verdenelli, Serri, Restovich, Andriulo, Meriles and Vargas-Gil2016) found that a mixture of oat, radish and vetch showed superiority in improving soil microbial community structure and phosphatase activity than the CC mixture that include oat and radish. In addition to the types of CC, the duration of CC also affects soil microbial activity and soil health. Short-term CC had variable effect on soil enzyme activities across locations, crop managements and soil conditions (Rankoth et al., Reference Rankoth, Udawatta, Veum, Jose and Alagele2019b). A one-year application of black oat CC increased soil microbial biomass, β-glucosidase and alkaline phosphatase activities than the NCC treatment (Zibilske and Makus, Reference Zibilske and Makus2009). Similar to this finding, a 3-year study indicated that a mixture of oat, radish and vetch significantly increased the soil PLFA and acid phosphatase activity than the NCC treatment (Chavarría et al., Reference Chavarría, Verdenelli, Serri, Restovich, Andriulo, Meriles and Vargas-Gil2016). Whilst, Calderon et al. (2016) found that in semiarid cropping system, the impact of one-year CC (regardless of single or multi-species) on microbial activities, soil microbial community composition and related enzyme activities was insignificant. However, long-term usage of CC generally demonstrated positive effects on soil microbial activity and nutrients availability (Almeida et al., Reference Almeida, Menezes-Blackburn, Zhang, Haygarth and Rosolem2019; Schmidt et al., Reference Schmidt, Gravuer, Bossange, Mitchell and Scow2018). Balota et al. (Reference Balota, Calegari, Nakatani and Coyne2014) suggested that soil microbial biomass, glomalin content, phosphatase and arylsulfatase activities were significantly improved by integrating CC for 23 years into the cropping systems. Similarly, it has been reported that glomalin content, AMF spores and its hyphae length were markedly increased after more than 5-year of barley application compared to the fallow (NCC) treatment (García-González et al., Reference García-González, Quemada, Gabriel, Alonso-Ayuso and Hontoria2018; García-González et al., Reference García-González, Quemada, Gabriel and Hontoria2016).
CC frequency in the crop rotations is one of the most important supporters of significant changes on soil enzyme activities. Data showed that CC planted annually rather than every few years lead to greater β-glucosidase, alkaline phosphatase and dehydrogenase activities (Brennan and Acosta-Martinez, Reference Brennan and Acosta-Martinez2017, Reference Brennan and Acosta-Martinez2019). Austin et al. (Reference Austin, Wickings, McDaniel, Robertson and Grandy2017) indicated that annually growing cover cropping had more potential to the accumulation of soil carbon and mitigation of soil degradation compared to every other year cover cropping system. The interactive impacts of CC for different durations under various cropping systems on soil health indicators are very limited. Furthermore, it is critical for producers to understand how soil health changes for crop rotations managed with and without CC so as to strategically plan their management. Therefore, the present on-farm study was conducted with the specific objective to assess the impacts of CC used for different durations (3–20 years) at five different environmental locations on selected soil biochemical properties and microbial community. This study can provide important information to producers regarding the crop types that they can adopt when considering the CC under no-till system depending upon the limited planting window after the cash crops. The selection of CC in the rotation of the study region was based on the following criteria: (i) different CCs represent the common grower situation in our region, and (ii) small planting window (period between cash crop harvest and planting CC) plays important role in using the CC. In our paper, producers of the region have been using CC in their crop rotations for different objectives such as for livestock grazing, economics, soil health, crop improvement and many others. Therefore, different farms were selected to include different rotations and durations of CC with the major objective to assess the impacts of CC on soil health.
Materials and methods
Study farms, management and treatments
This study was carried out at five farms, four producer farms and one experimental farm located in South Dakota and Iowa, USA. The South Dakota and Iowa states have a mean annual temperature of 6.9 and 8.5°C, and the mean annual precipitation is 522 and 895 mm, respectively. The farms included CC under different usage durations ranged from 3 to 20 years under different crop rotations. Each farm has CC and NCC treatments with three replications. The study design for the experimental farm (Site 5) was a randomized complete block design with three replications. The size of the plot at experimental site was 29 by 38 m, while the fields on producer farms were usually 20–25 ha in size. Information about the farm and cropping system is summarized in Table 1. The farms in this study were selected to represent the typical farming systems in the region covering a range of soil management practices in terms of crop diversity and CC application. Two fields (>20–25 ha) were selected on each farm in cooperation with an extension agent. Three farms (Sites 1, 3, and 4) had CS rotation, and CC was planted after maize harvest with CC growing at the time of sampling. Among the rest two farms one (Site 2) had CSWw rotation and the other (Site 5) had CO rotation, and CC were used after winter wheat and oats, respectively, in these rotations. Soil samples were collected during the CC phase.
Table 1. Basic information of cover crops (CC), soil type, location, crop rotation and average annual rainfall for the five study sites
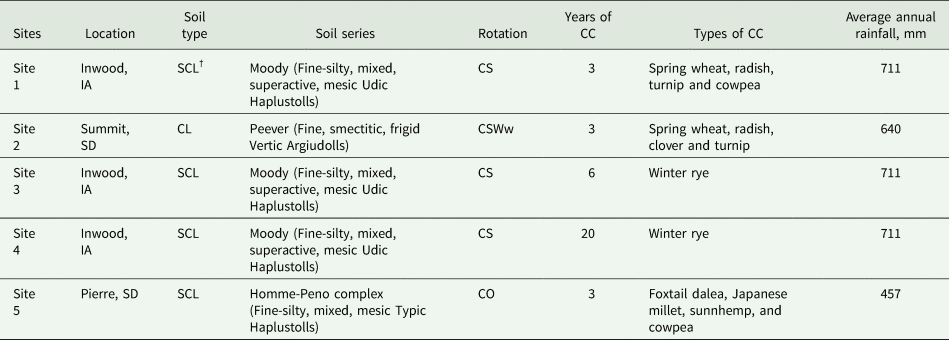
† SCL, silty clay loam; CL, clay loam; CS, maize–soybean; CSWw, maize–soybean–winter wheat, CO, maize–oat.
Soil sampling and analysis
Soil samples for 0–5 cm depth were collected using a spade from all the five farms during October 2018. The moisture content of the soil during the soil sampling was close to the field capacity. At each farm, the soil was collected from nine representative points within the field and pooled into a composite sample. However, farms of the producers were usually 20–25 ha in size, and we marked three pseudoreplicated areas of 10 m × 10 m with a similar landscape in individual field of all the four farms, collected nine soil samples from each pseudoreplicated area, and made one composite sample. Three replications were used for this study for every treatment. However, treatments at Site 5 were established under an experimental design at the producer farm with six plots, and soil samples were collected from each plot.
At each site (except Site 5), an additional sample was taken from a field managed with similar rotation but that included an NCC (size of the NCC was 20–25 ha), and was identified adjacent to the field (at least 1–5 m distance from the field edge, and at least 2 m distance from roads and tracks). The NCC field represents the traditional management that producers have been following in the region and served as benchmark for the potential soil health at each site. Samples from NCC field were collected in the same way as samples from the CC field. The sampling approach used in this study resembles that in various studies (e.g., Blanco-Canqui et al. Reference Blanco-Canqui, Stone, Schlegel, Lyon, Vigil, Mikha, Stahlman and Rice2009; dos Reis Ferreira et al. Reference dos Reis Ferreira, da Silva Neto, Pereira, do Nascimento Guedes, Rosset and dos Anjos2020; Sekaran et al. Reference Sekaran, Sagar, Denardin, Singh, Singh, Abagandura, Kumar, Farmaha, Bly and Martins2020, Reference Sekaran, Kumar and Gonzalez-Hernandez2021; Williams et al., Reference Williams, Colombi and Keller2020). All the soil samples were kept in a cooler during transportation and cold storage at 4°C pending analysis.
Soil pH, electrical conductivity and carbon and nitrogen fractions
Soil pH and electrical conductivity (EC) were determined using the pH and EC meter (Thermo Scientific Orion, model-Orion Star A215). Water-soluble C and N fractions were analyzed using cold water and hot water extraction methods (Ghani et al., Reference Ghani, Dexter and Perrott2003). Briefly, 3 g of soil and 30 ml of distilled water were added to a centrifuge tube and mixed with an end-to-end shaker at 40 rpm for 30 min. The resulting soil suspension was centrifuged at 3000 rpm for 25 min to isolate the cold water-extractable carbon (CWC) and nitrogen (CWN) in the supernatant. Thereafter, 30 ml of water was mixed with the remaining soil and placed in a hot water bath (80°C) for 12 h, and the suspension was centrifuged at 3000 rpm for 25 min to isolate the hot water-extractable carbon (HWC) and nitrogen (HWN). These soil extracts were analyzed with a TOC-L analyzer (Shimadzu Corporation, model-TNM-L-ROHS). Microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were determined using the chloroform fumigation extraction method outlined in Ross (Reference Ross1990). Ten grams of soil was fumigated with CHCl3 in a desiccator for 24 h and extracted with 40 ml 0.5 M K2SO4. Non-fumigated soils were treated similarly, and both the extracts were analyzed with the TOC-L analyzer. The MBC and MBN concentrations were determined by the calculating the difference between C and N in the fumigated and non-fumigated samples using an extraction efficiency factor of 0.45 (Beck et al., Reference Beck, Joergensen, Kandeler, Makeschin, Nuss, Oberholzer and Scheu1997). The result was expressed as mg/kg soil.
Soil enzymes
The β-glucosidase (EC 3.2.1.21) enzyme activity was assayed as described by Eivazi and Tabatabai (Reference Eivazi and Tabatabai1988). One gram of soil (<2 mm), 0.2 ml of toluene, 4 ml of modified universal buffer (MUB) pH 6.0, 1 ml of 50 mM p-nitrophenyl-β-D-glucosidase (PNG) solution were mixed in a flask and incubated at 37°C for 1 h. The reaction was ended by adding 1 ml of 0.5 M CaCl2, and 4 ml of 0.1 M tris (hydroxymethyl) aminomethane (THAM) buffer (pH 12). The soil suspension was filtered and the amount of p-nitrophenol (pNP) released was measured with a spectrophotometer at 405 nm. Control samples were included for each assay by the procedure described above, except adding the substrate PNG solution after using the THAM buffer (pH = 12). The result was expressed as μmol pNP/g soil/h. Urease (EC 3.5.1.5) activity was measured by colorimetric determination as described by Kandeler and Gerber (Reference Kandeler and Gerber1988). Five grams of soil was placed into each of three incubation flasks, 2.5 ml of 720 mM urea as substrate were added into the first two flasks and the third flask was a control. Twenty millilitre of borate buffer was added to all flasks; parafilm-sealed flasks were incubated at 37°C for 2 h. Released ammonium was determined with a spectrophotometer at 660 nm and expressed as μg N-NH4+/g soil/h.
Arylamidase (EC 3.4.11.4) activity was determined by the method described by Acosta-Martínez (Reference Acosta-Martínez2000). One gram of air-dried soil (<2 mm), 3 ml of 0.1 M THAM buffer (pH = 8), 1 ml of 8.0 mM L-leucine-β-naphthylamide hydrochloride (substrate) were combined in a flask and placed in an incubator-shaker at 37°C for 1 h. The intensity of the resulting red azo compound was measured with a spectrophotometer at 540 nm. The control samples received the 1 mL of substrate after incubation. The result was expressed as μg β-naphthylamine/g soil/h.
The method described by Eivazi and Tabatabai (Reference Eivazi and Tabatabai1977) was used to determine acid phosphatase (acid P) (EC 3.1.3.2) and alkaline phosphatase (E.C.3.1.3.1) (alkaline P) enzyme activities. One gram of soil (<5 mm), 0.2 ml of toluene, 4 ml of MUB (pH 6.5 for assay of acid phosphatase or pH 11 for assay of alkaline phosphatase), 1 ml of p-nitrophenyl phosphate solution were mixed, sealed, and incubated at 37°C for 1 h. After incubation, the reaction was terminated with 1 ml of 0.5 M CaC12 and 4 ml of 0.5 M NaOH, mixed, and the soil suspension filtered. The yellow colour intensity of the filtrate was determined with a spectrophotometer at 405 nm. The result was expressed as μg pNP/g soil/h. Arylsulfatase (EC 3.1.6.1) enzyme activity was determined according to the method described by Tabatabai and Bremner (Reference Tabatabai and Bremner1970). One gram of field-moist soil (<2 mm), 0.25 ml of toluene, 4 ml of acetate buffer, and 1 ml of p-nitrophenyl sulfate solution (0.05 M) were mixed and incubated 37°C for 1 h. After incubation, the reaction was terminated with 1 ml of 0.5 M CaC12 and 4 ml of 0.5 M NaOH, the soil suspension was filtered, and the yellow color intensity of the filtrate was measured with a spectrophotometer at 420 nm. The control sample was included for each assay by the procedure described above, except 1 ml of p-nitrophenyl sulphate substrate solution was added immediately before filtration of the soil suspension. The result was expressed as μg pNP/g soil/h.
Green et al. (Reference Green, Stott and Diack2006) method was used to determine the FDA hydrolysis activity in the soil. One gram of air-dried (sieved <2 mm) soil and 50 ml of THAM buffer (0.1 M, pH = 7.6) and 0.5 ml of 4.9 mM FDA lipase substrate solution was added to all flasks, except 0.5 ml acetone was added to the control sample instead of substrate solution. The contents were mixed, sealed and incubated at 37°C for 3 h. Two millilitres of acetone was added to all flasks after incubation and same amount of substrate solution was added to the control sample. The suspension was filtered and measured with spectrophotometer at 490 nm. The result was expressed as mg fluorescein/kg soil/h.
PLFAs and soil protein
PLFA analysis was conducted at Ward Laboratories Inc. (Lincoln, NE). Briefly, 2 g of lyophilized soil was used to extract the lipids with 9.5 ml dichloromethane: methanol: citrate buffer (1:2:0.8 v/v) extraction solution, then the above-extracted solution went through a solid-phase silica column to separate the phospholipids from other lipids. The extracted fatty acid methyl esters (FAMEs) were estimated with an Agilent 7890A GC equipped with a CP-7693 auto-sampler and a flame ionization detector. The following fatty acids were computed for specific notional groups: i-15:0, a-15:0, i-16:0, i-17:0 and a-17:0 were for gram-positive bacteria; 16:1w7, 17:0cy, 2-OH 16:0, c18:1w7 and 19:0cy were for gram-negative bacteria; 16:0 10-methyl, 17:0 10-methyl and 18:0 10-methyl for actinomycetes; 16:1w5 and 20:4w6 for AMF and 18:3w3, c18:2w9,12 and c18:1w9 for saprotrophic fungi. The 16:0 fatty acid and the general bacterial indicators 14:0, 15:0, 17:0 and 18:0 were considered as universal PLFA biomarkers. The PLFA 18:2o6c was used for fungal biomass and FAME 16:1ɷ5 to indicate AMF. The 16:1w5 biomarker can also be detected in gram-negative bacteria (Nichols et al., Reference Nichols, Mancuso and White1987). The bacterial PLFAs were calculated with FAMEs 3OH-12:0, a-12-meth-15:0, i-13-meth-15:0, 15:0, 2OH-14:0, i-14-meth-16:0, 16:1ɷ7c, i-15-meth-17:0, 10-methyl-17:0ɷ8c, 17:0 and 2OH-16:0 based on the bacterial standards used. The result was expressed as ng PLFA-C/g soil.
The soil protein content was analyzed based on the protocol from Wright and Upadhyaya (Reference Wright and Upadhyaya1996) and Hurisso et al. (Reference Hurisso, Moebius-Clune, Culman, Moebius-Clune, Thies and van Es2018). Briefly, 3 g of soil, 24 ml of sodium citrate buffer (20 mM, pH = 7) were added to a pressure and heat-stable glass screw-top tube and the contents were mixed within an end-to-end shaker at 90 rpm for 10 min. Then the tubes were autoclaved for 30 min (121°C, 15 psi) and thereafter cooled to room temperature. Two mL of the slurry was transferred to a smaller microcentrifuge tube and centrifuged at 10 000 g to remove soil particles. Then 0.1 ml subsample and 2 ml bicinchoninic acid were mixed and incubated at 37°C for 30 min and the protein concentration of the extract was determined with a spectrophotometer at 562 nm. Extractable protein content of the soil was calculated by multiplying the protein concentration of the extract by the volume of extractant used and dividing by number of grams of soil used. The data were expressed in mg/g soil.
Statistical analysis
Differences in soil properties between CC and NCC were analyzed using an analysis of variance in the SAS 9.4 (SAS, 2013). Mean values were separated using the least square means test when treatments were significant. Data were transformed using Box–Cox method as needed (Box and Cox, Reference Box and Cox1981). Statistical differences among treatments were stated significant at α = 0.05 level. The relationship between soil properties and biochemical and community structures was also determined by Pearson's correlation matrix using SAS version 9.4 (SAS, 2013).
Results
Soil pH, EC and water-extractable carbon and nitrogen fractions
The effect of different CC managements on soil pH and EC was significant (Table 2). The soil at all sites was weakly acidic, which ranged from 4.85 to 6.94. The soil EC ranged from 0.1 to 0.53dS/m. Results showed that soil pH and EC responses to CC managements vary at different farms. Soil pH under CC was decreased by 0.2 and 0.1 times compared with that under NCC treatment for Sites 1 and 3, respectively. No significant difference was observed between CC and NCC treatment on soil pH for Site 2. The CC treatment had 0.3 and 0.2 times higher soil pH than the NCC for Sites 4 and 5. The CC treatment affected soil pH and EC with identical trends at each site, viz. CC decreased soil EC at Sites 1 and 3, increased soil EC at Sites 4 and 5, but causes no differences at Site 2. The MBC and MBN contents as affected by different CC management at all sites are also summarized in Table 2. The MBC and MBN contents were higher in CC compared to the NCC treatments for all the farms, except for Site 1. There were no significant changes in MBC and MBN contents due to the CC management at Site 1. The MBC and MBN under CC treatment were 0.4, 0.5, 0.9, 0.9, 3.6 and 2.3 times greater than the NCC for Sites 2, 4 and 5, respectively. At Site 3, the MBC content under CC management was 0.9 times higher than that under NCC treatment. However, the CC treatment did not have a significant influence on MBN.
Table 2. Soil pH, electrical conductivity (EC), microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) as influenced by cover crop (CC) and no CC (NCC) management at 0–5 cm depth for all the sites.
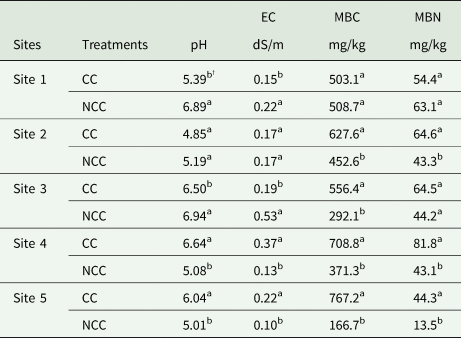
† Mean values within the same column followed by different small letters are significantly different at P < 0.05 between treatments for each site.
The impacts of different CC management practices on soil water-extractable C and N fractions are presented in Figs 1 and 2. CCs, in general, increased the CWC and CWN at all the sites, however, differences were not always significant. At Site 1, the concentration of CWC was significantly higher under NCC treatment than the CC. However, CWN was not affected by the CC at this site (Fig. 1). The CC treatment had 0.3, 0.8, 1 and 0.8 times higher CWC and CWN concentration compared to the NCC at Sites 2 and 5, respectively. No difference was detected between CC and NCC treatments in terms of CWC and CWN at Sites 3 and 4. CCs significantly increased the HWC compared with that of NCC for all the sites except Site 2. A similar trend was observed for the HWN except for Site 2. The CC increased the HWC and HWN by 0.4, 0.6, 0.4, 0.5, 1.3, 1.5, 1.5 and 1.8 times as compared to that of NCC treatment for Sites 1, 3, 4 and 5, respectively. However, for Site 2, the NCC had higher HWC and HWN contents compared with the CC treatment, although, the differences were not always significant.
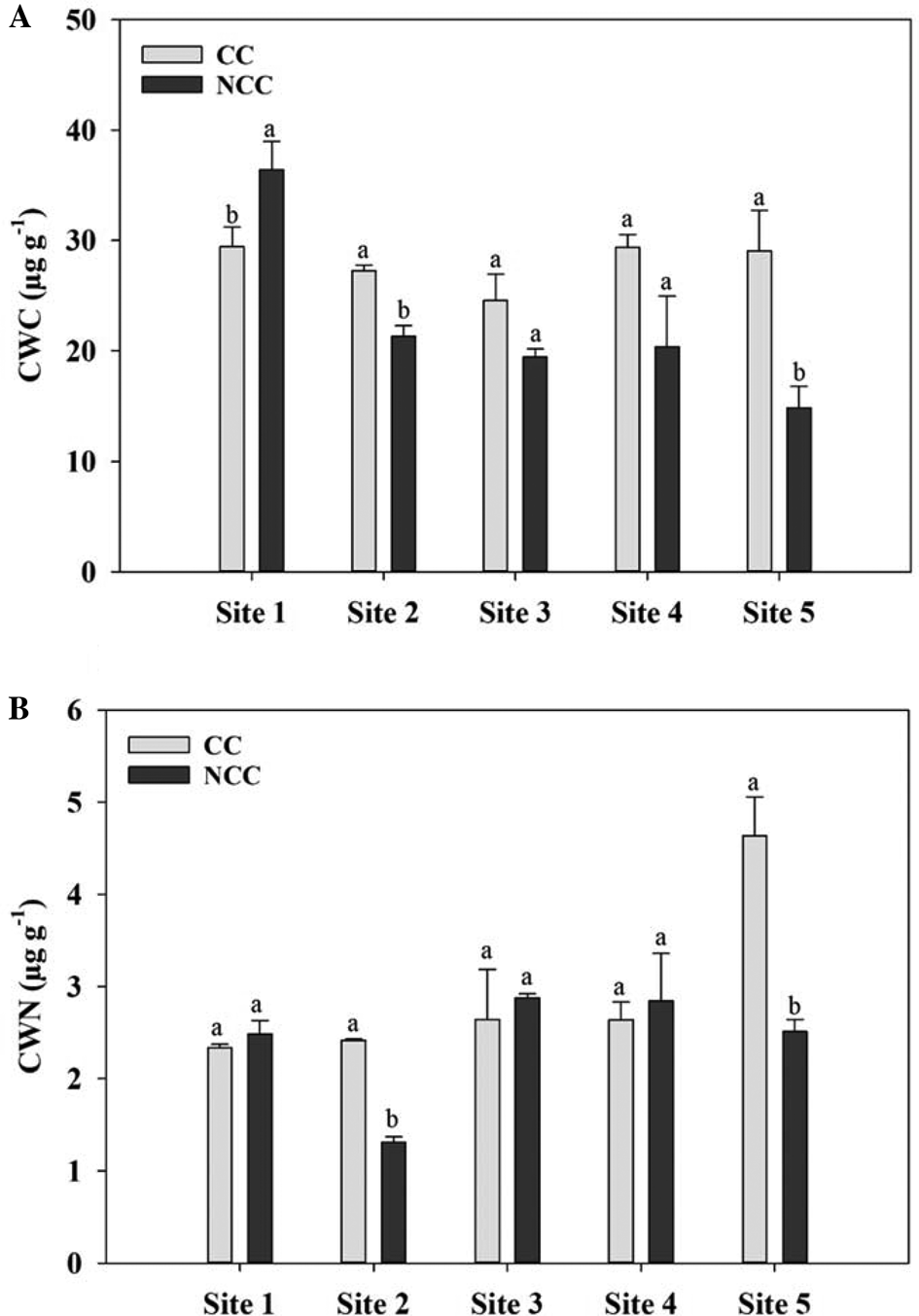
Fig. 1. Cold water-extractable carbon (CWC, A) and cold water-extractable nitrogen (CWN, B) contents at 0–5 cm depth under cover crop (CC) and no CC (NCC) treatments for five different study sites. Within the same site, different letters represent significant differences between treatments at P < 0.05.
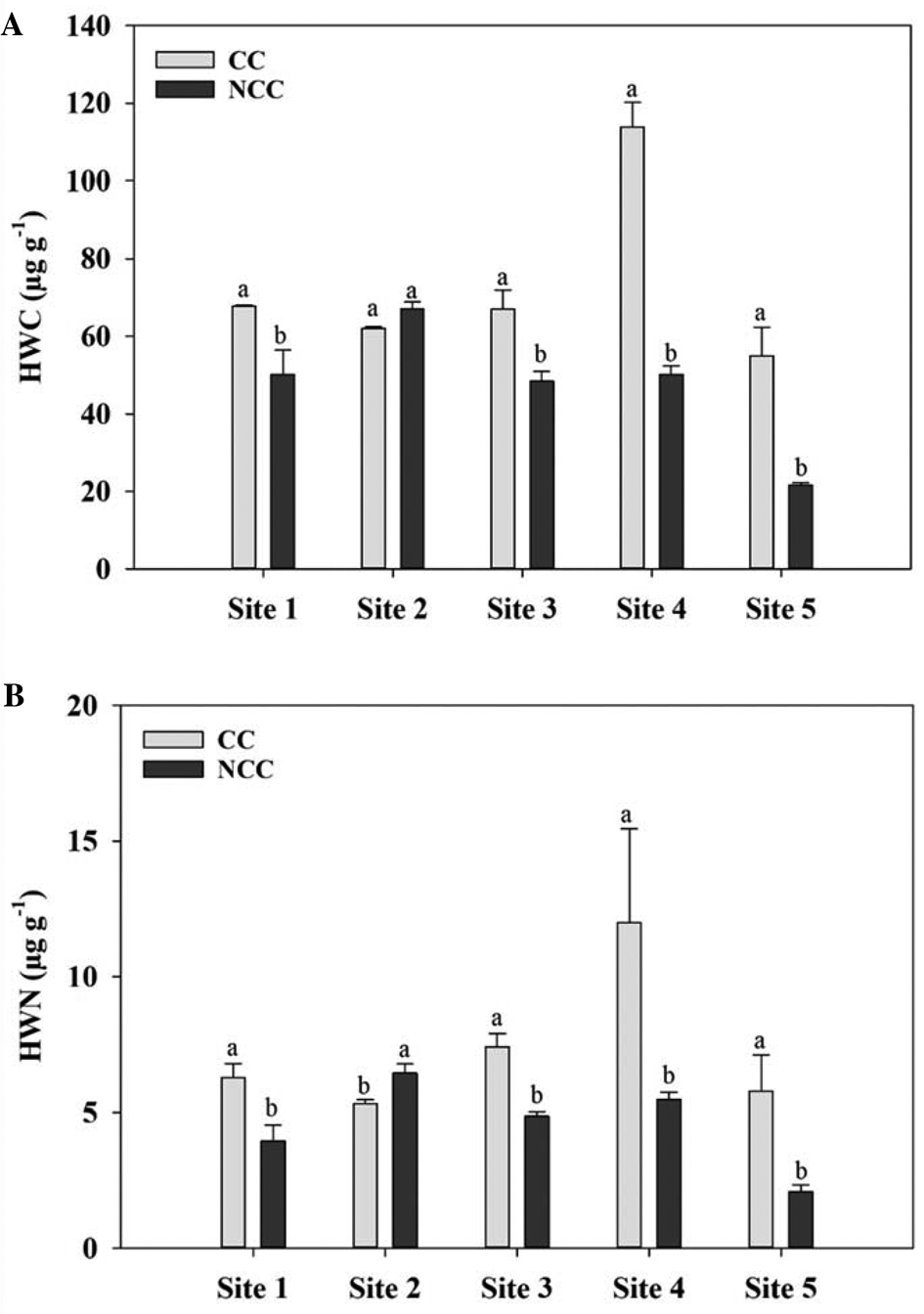
Fig. 2. Hot water-extractable carbon (HWC, A) and hot water-extractable nitrogen (HWN, B) contents at 0–5 cm depth under cover crop (CC) and no CC (NCC) treatments for five different study sites. Within the same site, different letters represent significant differences between treatments at P < 0.05.
Soil enzyme activities
Impacts of CC management on soil β-glucosidase, urease, arylamidase, acid phosphatase (acid P), alkaline phosphatase (alkaline P), arylsulfatase and FDA activities at 0–5 cm depth are shown in Table 3. In general, long-term (20-year) CC performed better in improving all enzymes activates. Data showed that there was a clear trend that CC at all sites had greater β-glucosidase content than NCC, however, the significant difference was only observed at Sites 4 and 5, where the β-glucosidase content under CC treatment was 1.2 and 0.9 times greater than that under NCC, respectively. The CC treatment significantly increased urease activity than the NCC for Sites 1, 3 and 4. However, no significant differences in urease activity were observed between CC and NCC treatments at Sites 2 and 5. The arylamidase activities were lower in CC than that in NCC treatment at Site 1, however, CC increased arylamidase activity compared to the NCC for Sites 4 and 5. The CC effect on arylamidase activity was not distinct at Sites 2 and 3, viz. the mean values of arylamidase activities were not statistically different in CC and NCC treatment. CCs significantly impacted the phosphorus cycling (acid P and alkaline P) at all sites except Site 1 (Table 3). At Site 2, the acid P and alkaline P under CC treatment were 0.2 and 0.4 times greater than those under NCC, respectively. At Site 3, the CC had a higher value of acid P compared with the NCC treatment, whereas, lower value of alkaline P was observed in CC treatment. The acid P and alkaline P activities were improved by 1.5, 4.1, 0.7 and 1.5 times with the addition of CC management practices compare to the NCC treatment at Sites 4 and 5, respectively.
Table 3. Soil enzyme activities as influenced by cover crop (CC) and no CC (NCC) management at 0–5 cm depth for all the sites
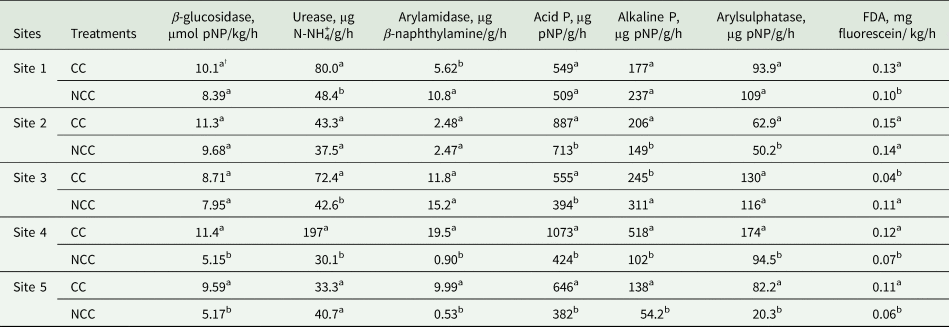
† Mean values within the same column followed by different small letters are significantly different at P < 0.05 between treatments for each site. FDA, Fluorescein Diacetate; P, Phosphatase.
With respect to arylsulfatase, there was an obvious trend that the arylsulfatase value was greater under CC treatment than that under NCC treatment at four out of five sites, although the difference was not significant at Site 3 (Table 3). At Sites 2, 4 and 5, the CC increased the arylsulfatase enzyme activity by 0.3, 0.8 and 3 times compared with NCC treatment, respectively. The FDA activity was increased under CC treatment at four out of five sites, and it was not statistically different for Site 2. The CC treatment recorded the greater FDA activity compared with NCC for Site 1 (0.3 times), Site 4 (0.7 times) and Site 5 (0.8 times), and lower activity for Site 3 (0.6 times).
PLFA and soil protein
Impacts of different CC management practices on soil PLFA analysis at 0–5 cm depth are presented in Table 4. Generally, the presence of CC improved total biomass, total bacterial, total fungi, gram-positive bacteria, gram-negative bacteria, AMF, actinomycetes and saprophytes in the soil compared to NCC, while the significant differences were only observed at Sites 4 and 5. In specific, no differences between CC and NCC in terms of all PLFA parameters were observed at Sites 1 and 3. All the PLFA parameters showed greater value under CC treatment compared with NCC treatment, only gram-positive bacteria were detected significantly increased under CC treatment compared to NCC treatment at Site 2. CC treatment at Sites 4 and 5 indicated greater total biomass, total bacterial, total fungi, gram-positive bacteria, gram-negative bacteria, AMF, actinomycetes and saprophytes as compared with NCC treatment. Higher soil protein contents were presented at most of the sites in this study, although the differences were not always significant. CCs increased soil protein content by 0.4, 0.5 and 0.8 times compared with NCC treatment at Sites 1, 3 and 4, respectively, but no significant differences between CC and NCC were observed at Sites 2 and 5 (Fig. 3).
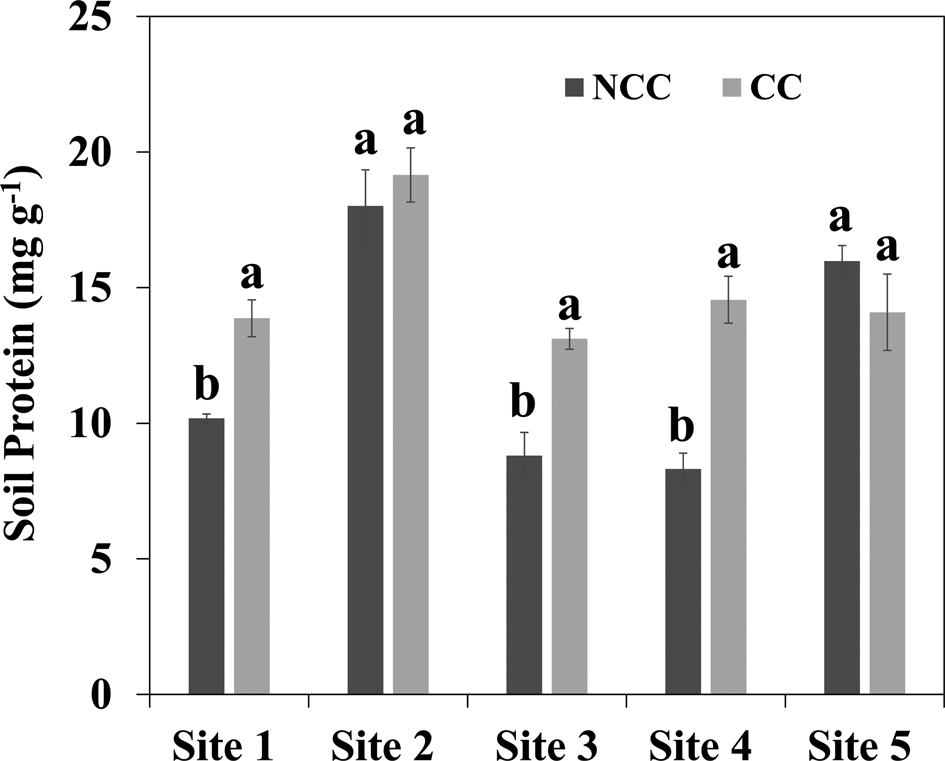
Fig. 3. Soil protein content at 0–5 cm depth under cover crop (CC) and no CC (NCC) treatments for five different study sites. Within the same site, different letters represent significant differences between treatments at P < 0.05.
Table 4. Total biomass, total bacterial, total fungi, gram-positive (+ve) bacterial, gram-negative (−ve) bacterial, arbuscular mycorrhizal fungi (AMF), actinomycetes, and saprophytes PLFA (phospholipid fatty acid) biomass as influenced by cover crop (CC) and no CC (NCC) management at 0–5 cm depth for all the sites
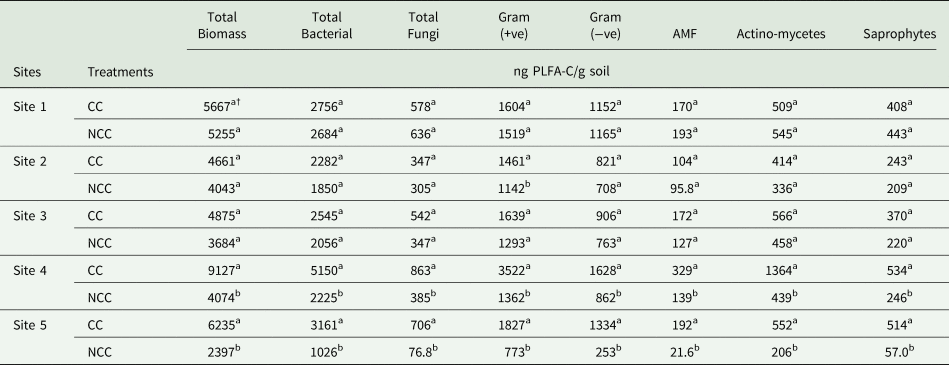
† Mean values within the same column followed by different small letters are significantly different at P < 0.05 between treatments for each site.
Pearson correlation analysis
Correlation analysis showed soil physicochemical properties were positively and significantly correlated with the soil enzyme activities (P < 0.05) and microbial communities (P < 0.01) (Fig. 4). Soil pH was significantly correlated with total bacterial, arylamidase, AMF (P < 0.01), urease and gram-positive bacterial PLFA contents (P < 0.05). Soil EC was significantly correlated with total bacterial, arylamidase, AMF, gram-positive bacterial PLFA contents (P < 0.05). More than 63% of the variation in the arylamidase activity can be explained by the variation of soil pH, EC and HWN content. The content of CWC and soil MBC showed a positive correlation with soil enzyme activities (P < 0.05) and PLFA profiles (P < 0.01). The content of HWN was significantly correlated with soil enzyme activities and PLFA biomasses (P < 0.01). There were no selected soil physicochemical properties significantly correlated with soil protein content.
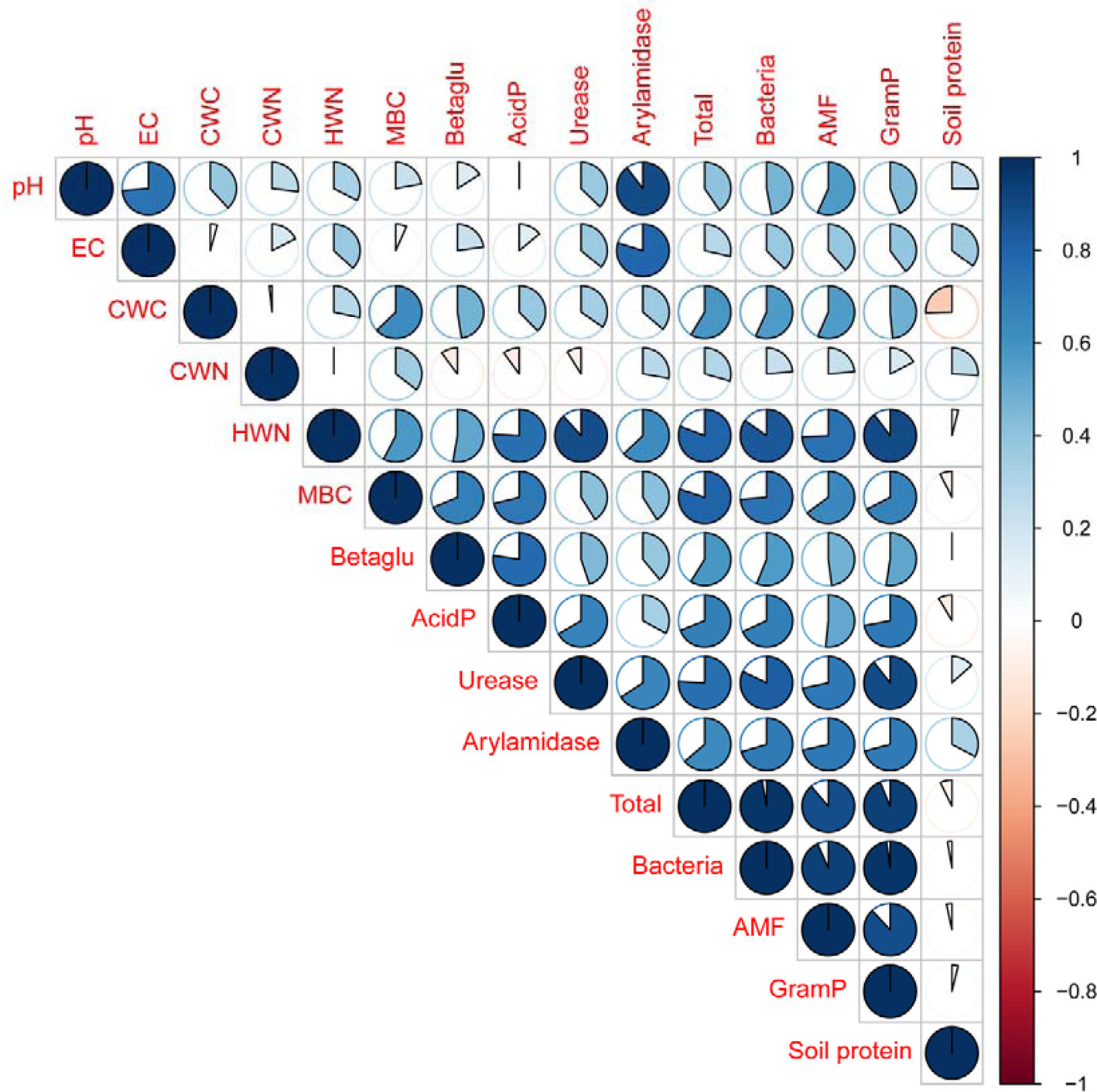
Fig. 4. Pearson correlation analysis between soil physicochemical properties and microbial properties. Note: EC, electrical conductivity; CWC, cold-water extractable carbon; CWN, cold-water extractable nitrogen; HWN, hot-water extractable nitrogen; MBC, microbial biomass carbon; Betaglu, β-glucosidase; Acid P, acid phosphatase; Total, total biomass; AMF, arbuscular mycorrhizal fungi and GramP, Gram-positive bacterial.
Discussion
The present study was conducted on five producer farms, which helped us to consider various CC used in maize-based cropping systems under different locations with the maize–soybean as the major crop rotation of the region. Compared with the experimental farm only design, the on-farm design helped to include large variations of the cover cropping duration in the maize-based cropping systems. Soil data collected in this study were compared for the individual sites at different treatments, yet we did not compare the data for different cropping systems or across the sites. Producers in the region adapt CC from year to year depending upon the crop rotations, soil conditions, weed pressure and the weather. Soil moisture is the prime deciding factor for the use of CC in the study region. Producers of the study region started showing an increased interest in growing multispecies CC in traditional maize and soybean rotations. Our findings showed the CC significantly affects the soil health, and that longer duration with no-till systems enhances the soil health.
CC influences on soil pH, EC and labile C and N fractions
Data showed that CC caused a reduction in soil pH and EC at Sites 1 and 3 but increased these soil parameters for Sites 4 and 5, and no distinctions at Site 2. Variations in soil pH and EC at all sites except Site 2 presumably were due to the addition of CC which can alter the soil organic matter content, and different decomposition rate under various cropping systems with dissimilar weather condition; different species of CC capture the different amount and types of nutrients from the soil and, therefore resulting in quantity changes in soil cation and anion (Vanzolini et al., Reference Vanzolini, Galantini, Martínez and Suñer2017). No significant changes in soil pH and EC at Site 2 may be attributed to the higher natural buffer capacity. Studies conducted on a sandy loam soil in Denmark reported by Abdollahi and Munkholm (Reference Abdollahi and Munkholm2014) and conducted on a silt loam soil in Alabama reported by Nyakatawa et al. (Reference Nyakatawa, Reddy and Sistani2001) showed that the application of 5-year fodder radish and 2-year winter rye CC did not impact soil pH, which may contribute to the short duration or the wider soil buffer capacity and stronger soil self-regulating ability. Similar result was reported by Sharma et al. (Reference Sharma, Irmak and Padhi2018) that a 3-year CC mixture planted on silt loam soil in Nebraska did not impact the soil pH and EC at 0–5 cm depth.
Data showed that CC enhanced the soil MBC, MBN, CWC, HWC and HWN contents for four out of five farm locations, although differences were not always statistically different. Microorganisms make a great contribution to the decomposition of organic matter and the release of plant-available nutrients (Schmidt et al., Reference Schmidt, Gravuer, Bossange, Mitchell and Scow2018). The MBC and MBN are the most labile living C and N of soil organic matter and work as an early indicator of changes in soil C and N (Mbuthia et al., Reference Mbuthia, Acosta-Martínez, DeBruyn, Schaeffer, Tyler, Odoi, Mpheshea, Walker and Eash2015; Moore et al., Reference Moore, Klose and Tabatabai2000). The CC recorded significantly higher MBC and MBN than the NCC treatment in four out of the five study sites, which attributed to the relatively higher biomass (personal communication from producer) on the soil surface and belowground. Our results are consistent with Zhu et al. (Reference Zhu, Yi, Guo, Chen, Hu, Tang, Xiao, Xiao, Yang and Acharya2012) who reported that the MBC and MBN were significantly increased by the presence of CC than the NCC treatment. Water-extractable C and N are the fractions of labile C and N pools, respectively, those are easily available for microorganisms (Gregorich et al., Reference Gregorich, Liang, Drury, Mackenzie and McGill2000; Sparling et al., Reference Sparling, Vojvodić-Vuković and Schipper1998). The higher water-extractable C indicated the higher available labile C and greater organic matter turnover. Results from this study showed that CWC and CWN values were increased by the presence of a mixture of CC (e.g., at Sites 2 and 5), because the diverse residues from these CC contribute more labile C and N to the soil. The inclusion of CC enhanced the HWC and HWN than the NCC treatment for all the study sites except for Site 2.
CC impacts on soil enzyme activities
CCs are helpful in altering soil microbial and enzyme activities (Frasier et al., Reference Frasier, Noellemeyer, Figuerola, Erijman, Permingeat and Quiroga2016; Sanchez et al., Reference Sanchez, Fultz, Lofton and Haggard2019b). The activity of soil enzymes can be characterized and reflect the microorganism activity and soil quality condition (Benintende et al., Reference Benintende, Benintende, Sterren and De Battista2008; Das and Varma, Reference Das and Varma2010). Results from this study showed that short-term cover cropping (Sites 1, 2, and 3) did not alter the β-glucosidase activities. However, the long-term cover cropping system (e.g., at Site 4) and short-term cover cropping system with legume and grass CC mixture (e.g., at Site 5) increased the β-glucosidase activities compared with the NCC treatment, which also correlated with the MBC. A similar finding was reported by Bandick and Dick (Reference Bandick and Dick1999) who showed that after integrating CC into the agricultural system for 6 years, the β-glucosidase activity was increased in cereal or legume CC treatment than the winter fallow or continuous fescue due to the increased C inputs from CC which can stimulate the microbial activity. Similarly, Dinesh et al. (Reference Dinesh, Suryanarayana, Chaudhuri and Sheeja2004) revealed that β-glucosidase activity was noticeably enhanced by including a 10-year leguminous CC which may attribute to the diversified root system and increased soil organic matter.
Microbial and enzymatic decomposition and mineralization of organic matter can provide the nutrients (N, P and S) for plant growth and production (Hai-Ming et al., Reference Hai-Ming, Xiao-Ping, Wen-Guang, Ye-Chun, Ke and Guang-Li2014). For the N-related urease and arylamidase activities, there was a general trend that CC increased the urease activities than the NCC, except not statistically different at Sites 2 and 5. Arylamidase activities for the short-term cover cropping system were not consistent, whereas, higher arylamidase activities were shown in the NCC at Site 1, and no difference between treatments at Sites 2 and 3. Long-term cover cropping (Site 4) and short-term cover cropping system with legume and grass CC mixture (Site 5) significantly enhanced the arylamidase activities that may attribute to the positive correlation with the HWN. Similar results were also reported by Bandick and Dick (Reference Bandick and Dick1999) who showed that cereal rye CC increased the urease activity, while legume CC (cereal rye/Austrian winter pea or red clover mix) did not enhance it as compared to the winter fallow, which may associate with species of CC. A study showed that the adoption of either crimson clover, black oat, or the mixture of crimson clover and black oat CC treatment significantly enhanced the arylamidase enzyme activity as compared to the NCC at 0–5 cm depth (Hamido and Kpomblekou-A, Reference Hamido and Kpomblekou-A2009). However, the urease activity was greater under crimson clover as compared to the soil from the NCC treatment in no-till system, while the black oat and the mixture of black oat and crimson clover did not cause significant changes in urease components (Hamido and Kpomblekou-A, Reference Hamido and Kpomblekou-A2009). Dinesh et al. (Reference Dinesh, Suryanarayana, Chaudhuri and Sheeja2004) indicated that urease and arylsulfatase activities were markedly enhanced by integrating 10-year leguminous CC due to the increased soil organic matter, which can provide more energy source and a more suitable microbial environment for the activity of microbes.
Data from P cycling-related enzymes showed that, in general, the content of acid P was several-fold greater than that of alkaline P for all the sites, which may be associated with the soil pH. Our results are in agreement with the study of Šarapatka et al. (Reference Šarapatka, Dudová and Kršková2004) who demonstrated that acid P activity negatively correlated with the soil pH. CC increased the acid P activity than the NCC for all the sites except for Site 1 where no differences in this activity were observed between CC v. NCC treatments. There was a notable increase in alkaline P due to long-term CC and short-term legume and grass CC mixture (Site 5) compared with the NCC treatment. This may be due to the reason that increased crop residue from CC left on the soil surface promotes the transformation of organic matter into mineral P and increased the P availability (Singh et al., Reference Singh, Bhattacharyya, Das, Sharma, Ghosh, Das and Jha2018). The trend for arylsulfatase enzyme activity was similar to the alkaline P, except the difference between treatments was not significant at Site 3. This can be partially attributed to the higher turnover of organic sulphur presented in CC than the NCC treatment. The CC had a profound effect on FDA at all the sites except Site 2 where the short-term cover cropping systems did not show consistent results regarding the FDA. Significant increase in FDA due to long-term cover cropping and short-term legume and grass CC mixture (Site 5) compared with NCC treatments could be attributed to diversified crop roots exudates released to the soil and greater decompose ability of organic P compounds generated by the inclusion of CC. This finding was consistent with a study conducted in Salta, Argentina by Brandan et al. (Reference Brandan, Chavarría, Huidobro, Meriles, Brandan and Gil2017) who demonstrated that the FDA and acid phosphatase activities were enhanced by including a 6-year of Brachiaria brizantha as CC than the NCC treatment which may contribute to the greater mineralization of organic matter rate and enhanced roots exudates through the implementation of CC. Similar results were also reported by Chavarría et al. (Reference Chavarría, Verdenelli, Serri, Restovich, Andriulo, Meriles and Vargas-Gil2016) where they showed an increase in FDA and acid phosphatase activities through the application of a 3-year CC mix (oat/radish and oat/radish/vetch) as compared to the NCC treatment with a Luvic Phaeozem dominated soil under the no-tillage system.
CC impacts on soil microbial community structure
Short-term (Sites 1, 2, and 3) cover cropping system did not cause any significant variations in the PLFA parameters (total biomass, total bacterial, total fungi, gram-negative bacterial, AMF, actinomycetes and saprophytes), except that a higher gram-positive bacterial value was recorded in CC treatment as compared to the NCC. There was a significant increase in all PLFA parameters due to the application of long-term cover cropping system (Site 4) and short-term legume and grass CC mixture (Site 5) in comparison with the NCC treatment, which may associate with the long-term continuous C input from the CC root exudates and crop residue. The person correlation analysis results showed there was a positive correlation between labile C (MBC and CWC) and total PLFA biomass. The increment of C content by the presence of CC could enlarge the abundance of soil microbial biomass. Similarly, Schmidt et al. (Reference Schmidt, Gravuer, Bossange, Mitchell and Scow2018) reported that CC results in a higher increase in total bacterial and make changes in the soil microbial community. Studies indicated that the abundance of total FAME, gram-positive bacteria, gram-negative bacteria, actinomycetes, AMF, saprophytic fungi, and protozoa were improved significantly at 0–5 cm depth under flax, oat, pea rapeseed or 10-species CC mixture as compared with control (NCC) at CC termination which attributes to the living roots stimulate the microbial activity (Calderon et al., Reference Calderon, Nielsen, Acosta-Martinez, Vigil and Drew2016). Compared to the NCC treatment, Chavarría et al. (Reference Chavarría, Verdenelli, Serri, Restovich, Andriulo, Meriles and Vargas-Gil2016) found that the total bacterial PLFA and the abundance of gram-positive bacteria were significantly increased in continuous soybean and soybean–maize rotations after a 3-year CC mix (oat/radish and oat/radish/vetch) application in Buenos Aires, Argentina. Similarly, Mbuthia et al. (Reference Mbuthia, Acosta-Martínez, DeBruyn, Schaeffer, Tyler, Odoi, Mpheshea, Walker and Eash2015) also reported that the richness of gram-positive bacteria was greater by including the legume CC hairy vetch than the control (NCC) treatment which may contribute to the legume CC hairy vetch increased N and C concentration to the soil. Similar results were also found in a study conducted on sandy loam soil in La Pampa, Argentina which showed a significant increase in gram-positive bacteria under either rye CC or a mixture of rye and vetch CC relative to the NCC treatment, but the abundance of total bacterial, fungi and gram-negative bacteria were almost similar among all the treatments (Frasier et al., Reference Frasier, Noellemeyer, Figuerola, Erijman, Permingeat and Quiroga2016).
CC impacts on soil protein
CCs increased the soil protein content compared with the NCC treatment for all the sites, however, significant differences were observed only at Sites 1, 3, and 4. Higher soil protein content under CC treatment may be caused by stimulation of microbial activity with the long-term presence of CC. Besides the length of CC cultivation, the enhancement of soil protein also associated with the species of CC; grass CC (barley) increased the quantity of GRSP than the NCC treatment, but not for legume CC (vetch) (García-González et al., Reference García-González, Quemada, Gabriel, Alonso-Ayuso and Hontoria2018, Reference García-González, Quemada, Gabriel and Hontoria2016). Besides, released anti-fungal compounds from Brassicaceae (radish and turnip) roots impeded the production of GRSP by AMF can be another reason to minimize the differentiation in soil protein content between CC and NCC treatments (Higo et al., Reference Higo, Gunji and Isobe2017).
In general, short-term winter rye and CC mixture at Sites 1, 2 and 3 did not show a consistent effect on soil enzyme activity and PLFA biomass. This may be partially attributed to various reasons that include: different weather and moisture contents at each site, different fertilizer management at these sites causing different C:N ratios and leading to different decomposition rate, different types of CC and different CC management (seeding rate, date and depth) resulting different amount of biomass and allow CC have different duration to decompose and release nutrients (Romdhane et al., Reference Romdhane, Spor, Busset, Falchetto, Martin, Bizouard, Bru, Breuil, Philippot and Cordeau2019). The long-term winter rye CC increased the soil enzymes, soil community structure and abundance, and soil protein content by producing large amounts of residue and increasing AMF (Finney et al., Reference Finney, Buyer and Kaye2017). The short-term legume-grass CC mixture at Site 5 had a similar effect trend as long-term winter rye CC which may contribute to the increased N in the soil enhance the decomposition rate and shorter the OM turnover rate.
On-farm research is more representative of the realistic situation than university-based small plot research. It makes the effect of changing practice more intuitive for farmers and it is a more persuasive tool to influence their future decision and operation. The result of this on-farm scale research also provide real and effective information to local farmers who want to improve soil health in this area, and it will be useful for farmers better manage their land. Our results have proved that long-term planting of CC has more obvious and detectable effects on soil health. In addition, adopting more replication in a structured way would be better to enhance the convincing and trustworthiness of the experiment if the field conditions allowed in the future field experiment.
Conclusions
This study investigated the impacts of CC management used in different cropping rotations on soil health indicators at five different farms. This on-farm study included various CC practices in the real-farm settings to include various cropping systems and the duration of the CC that are common in the study region. We observed that CC significantly enhanced soil health indicators, however, major significant impacts were observed when CC was used for at least six or more years.
Data showed that long-term cover cropping (>6 years) generally improved all the selected enzyme activities which involve in C, N, P and S cycling, shifted microbial community structure to wider metabolic capacities, and promoted the labile C and N turnover, and the soil microbial biomass. We reported that the soil health of the farms with CC was improved compared to the farms that did not use CC. CCs (winter rye, legume and brassica mixture) for shorter duration (3–6 years) did not cause any significant changes in the microbial community structure. Similarly, the enzyme activities, microbial biomass, C and N fractions response to the short-term cover cropping practices were not consistent. Our study showed that CC incorporation and the diverse rotations are the key to soil health, and suggest a higher crop diversity, use of no-till systems for enhancing the soil health. This study provides compelling evidence that a long-term application of winter rye CC or multispecies legume-grass CC mixture can have a beneficial impact in enhancing soil microbial activities and promoting the C, N, S and P cycling, and hence, these CC can be adopted in the Midwest US to improve the soil biochemical properties and the soil health. The relationship between the duration of CC and the types of CC is expected to examine in a systematic way in future research to improve the versatility of CC planting.
Acknowledgements
We thank the US Geological Survey, South Dakota Cooperative Fish & Wildlife Research Unit for administrative assistance with the research work order (RWO 116) at South Dakota State University. We also thank Dr. Mike Lehman and Dr. Shannon Osborne for their editorial comments on the manuscript.
Financial support
Financial support for this work was provided by the US Department of Agriculture, Natural Resources Conservation Service (grant no. G17AC00337) and the SD Agricultural Experiment Station (grant nos. H351-09 and H543-15).
Conflict of interest
None.
Ethical standards
Not applicable












