Introduction
The amphibian crisis has been widely recognized since the first World Congress of Herpetology in 1989 (Wake, Reference Wake1998). Habitat destruction, climate change and emerging infectious diseases, such as chytrid fungus Batrachochytrium dendrobatidis, have led to unprecedented rates of declines and extinctions (Whittaker et al., Reference Whittaker, Koo, Wake, Vredenburg and Levin2013; Scheele et al., Reference Scheele, Pasmans, Skerratt, Berger, Martel and Beukema2019). Over 40% of amphibian species are threatened with extinction and 128 categorized as Possibly Extinct (IUCN, 2017). Recognizing the need to have a ‘response that is at the scale of the challenge’ (Gascon et al., Reference Gascon, Collins, Moore, Church, McKay and Mendelson2007, p. 2), the IUCN Species Survival Commission coordinated global action between partner organizations through the Amphibian Conservation Action Plan in 2007, subsequently updated in 2015 (Wren et al., Reference Wren, Angulo, Meredith, Kielgast, Dos Santos and Bishop2015).
Captive breeding programmes are promoted by the Amphibian Conservation Action Plan to supply assurance populations for species facing rapid declines not preventable by in situ measures alone. The Amphibian Ark was established to facilitate captive breeding programmes in 2006 (Zippel et al., Reference Zippel, Johnson, Gagliardo, Gibson, McFadden and Browne2011). Since 1966, amphibian captive breeding and reintroduction programmes have been highly or partially effective in establishing 27 self-sustaining populations in the wild and there has been a 57% increase in species held in these programmes during 2007–2013 (Harding et al., Reference Harding, Griffiths and Pavajeau2016). Despite this increase, only 180 of the 577 species recommended for ex situ programmes by the Conservation Needs Assessment process (Conservation Needs Assessment, 2019) are currently held in captive breeding programmes. A further 69% of species remain to be assessed (Baker et al., Reference Baker, Johnson and Carrillo2017).
Historically, captive breeding programmes have been promoted as relatively simple and cost-efficient for amphibian conservation, but this perception overlooks the diverse environmental requirements of individual species that can render captive breeding programmes complicated and resource-intensive (Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015). The majority of species requiring such programmes are from the tropics, where diversity and endemism are greatest and threats most severe (Hof et al., Reference Hof, Araújo, Jetz and Rahbek2011). Many have poorly studied life histories, posing additional challenges in learning how best to keep and breed them in captivity (Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015). Furthermore, little is known about programmes operating in the tropics compared to the Western world (Brady et al., Reference Brady, Young, Goetz and Dawson2017). There are numerous benefits to operating programmes within species’ range countries (Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015; Pessier & Mendelson, Reference Pessier and Mendelson2017), but this often necessitates supporting programmes in less developed countries (Zippel et al., Reference Zippel, Johnson, Gagliardo, Gibson, McFadden and Browne2011), sometimes in politically and economically complex situations. To be effective, programmes must be designed to overcome a number common of issues by developing strategic partnerships to build capacity within range states (Meredith et al., Reference Meredith, St. John, Collen, Black and Griffiths2017).
Zoos and aquariums (hereafter zoos) are important for captive breeding programmes and other conservation initiatives, both ex situ and in situ (WAZA, 2005). Although the number of amphibian species held in zoos along with the proportional representation of globally threatened species has increased significantly during 1994–2014 (Dawson et al., Reference Dawson, Patel, Griffiths and Young2016), amphibians remain underrepresented in zoos compared to other vertebrate taxa (Conde et al., Reference Conde, Colchero, Gusset, Pearce-Kelly, Byers and Flesness2013). The funding, expertise and commitment to conservation provided by zoos make them key partners for programmes within species' geographical range that have low existing capacity for captive breeding (Harding et al., Reference Harding, Griffiths and Pavajeau2016; Griffiths, Reference Griffiths2017). In addition, zoos contribute through the keeping of threatened species in-house (Zippel et al., Reference Zippel, Johnson, Gagliardo, Gibson, McFadden and Browne2011; Dawson et al., Reference Dawson, Patel, Griffiths and Young2016; Brady et al., Reference Brady, Young, Goetz and Dawson2017).
Several factors hamper effective captive breeding programmes. Lack of resources (mainly space, staff time and budget), expertise and management interest is a major barrier to keeping threatened amphibians in zoos in developed countries (Barber & Poole, Reference Barber and Poole2014; Brady et al., Reference Brady, Young, Goetz and Dawson2017). O'Rourke (Reference O'Rourke2014) noted that captive breeding programmes are usually led by natural scientists, who tend to focus on biological success factors over social and political ones, although all those factors influence the effectiveness of captive breeding. The impacts of human dimensions such as cognitive biases and decision-making are widely recognized in many non-conservation sectors (Helmreich, Reference Helmreich2000; Edmondson, Reference Edmondson2011; Hickey et al., Reference Hickey, Pham-Hung, Nosikova, Halvorsen, Gritti and Schwartz2017; see review by Catalano et al., Reference Catalano, Redford, Margoulis and Knight2018). Despite the importance of organizational culture, institutional systems and other human factors in determining the effectiveness of organizations, these barriers have rarely been examined in the captive breeding literature (Sutton, Reference Sutton2015).
To improve our understanding of captive breeding programmes, we must examine the people and management practices that drive them, as well as the social-ecological systems within the regions in which they occur. Effective programmes should be systematically and strategically designed to overcome persistent challenges of conserving wild populations (Pritchard et al., Reference Pritchard, Fa, Oldfield and Harrop2012; IUCN, 2013). During 2009–2014, the Amphibian Ark's Conservation Needs Assessment (Conservation Needs Assessment, 2019) process was implemented to include characteristics beyond IUCN threat status and range, namely the ability of conservation practitioners to mitigate known threats, availability of protected habitat, scientific and cultural significance, suitability for husbandry analogue programmes, and availability of founding populations (Johnson et al., Reference Johnson, Baker, Buley, Carrillo, Gibson and Gillespie2018). Programme planning often lacks the inclusion of human, social and institutional factors that determine the programme's effectiveness.
Here, we aimed to identify barriers and enablers, in particular human and organizational barriers, of amphibian captive breeding programmes in Latin America, Africa and Asia. We provide recommendations for increasing the capacity and effectiveness of programmes in priority regions through partnerships and support.
Methods
Interviews with programme managers
The Amphibian Ark supports and monitors captive breeding programmes globally, and their database (Amphibian Ark, 2019) logs milestones for individual programmes along with institution and contact information. Complementing the Amphibian Ark database with our knowledge, we identified 50 programmes in Latin America, Africa and Asia that were established for the conservation of one or more species in country, or using analogous species to develop husbandry protocols for a more threatened species. We defined a practitioner (or manager) as any person involved in the design and implementation of a programme. All research and conservation programmes at a single institution were considered as one programme. We approached and interviewed managers using methods recommended by Dillman et al. (Reference Dillman, Smyth and Christian2014), in English or Spanish. We aimed to complete 20 interviews, to meet recommendations for in-depth, exploratory interview studies (Brinkmann & Kvale, Reference Brinkmann and Kvale2015).
We identified four research themes through consultations with key experts from Durrell Wildlife Conservation Trust, the Amphibian Ark and a former programme manager: (1) barriers and enablers, (2) partnerships, (3) progress of programmes and (4) programme structure. We designed a semi-structured interview guide (Supplementary Material 1) to address each theme using an inductive (qualitative) methodology, complemented with quantitative questions to address key aspects of the research, such as budgets and number of partnerships (Newing, Reference Newing2010).
We conducted three pilot interviews to develop a final interview guide containing interviewer prompts and definitions (Dillman et al., Reference Dillman, Smyth and Christian2014). Managers interviewed during the pilots were subsequently contacted with additional questions after piloting, allowing participants to expand on their initial responses. These interviews were included in the analysis to increase the sample size.
Interviews were conducted by BK in May and June 2017, primarily in English, with six interviews conducted in Spanish by a trained assistant, and one interview conducted with a Chinese translator. The interviews were semi-structured, allowing respondents to emphasize topics important to their work, whilst exploring new themes (Brinkmann & Kvale, Reference Brinkmann and Kvale2015). The interviews were audio recorded and transcribed verbatim.
Assessment of captive breeding programmes
We collected quantitative data on the organizational structure of programmes to explore the context of each programme and the commonalities and differences potentially influencing outcomes. Managers identified the three most important partners involved in their programme, and the resources they provided, irrespective of their effectiveness. These partners were grouped into types (with the exception of Amphibian Conservation Action Plan partners because of their focus on amphibian conservation) and tallied.
We assessed the progress of programmes against five criteria from the Amphibian Ark progress indicators (Amphibian Ark, 2019): (1) production of offspring, (2) production of viable second generation offspring, (3) reintroduction of captive-bred individuals into the wild, (4) if applicable, positive impact of reintroduced individuals on the wild population, and (5) monitoring of wild populations of captive-bred species (both reintroduced and native populations) by the programme or its partners. Because we analysed the programmes at an institutional level, a criterion was considered achieved if at least one species qualified. Two programmes did not keep amphibians and were only included in the thematic analysis. Data on programme structure for three programmes was lost through faulty audio recordings, but information on programme progress and partners was retained for all.
Thematic analysis
We analysed qualitative data under the barriers and enablers, and partnerships themes following the framework method (Gale et al., Reference Gale, Heath, Cameron, Rashid and Redwood2013; Table 1, Supplementary Material 2). The analysis identified barriers and enablers of programmes, and through examination of partnerships we assessed the drivers and constraints of these programmes. Barriers and enablers were defined as any material or non-material infrastructure, equipment, activity, plan, skill or any other factor influencing the functioning of a project, and a partnership was defined as any relationship providing external support for the programme. We analysed interview transcripts using NVivo 11 Plus (QSR International, Doncaster, Australia). Four audio recordings were faulty, leaving a written summary for analysis. Three additional e-mails from managers were included. The quality of the process was reported following Tong et al. (Reference Tong, Sainsbury and Craig2007; Supplementary Material 3a).
Table 1 Detailed analysis methodology using the framework method, following the seven steps described by Gale et al. (Reference Gale, Heath, Cameron, Rashid and Redwood2013).

The emerging analytical framework highlighted commonalities in barriers and enablers but not their relative importance (Supplementary Table 1). The three most important barriers for each programme were subjectively assessed by BK. Summaries were e-mailed to managers to confirm the interpretation. We categorized barriers according to the analytical framework and tallied them to identify the most frequent critical barriers and enablers (Supplementary Material 4).
Results
We had a 50% response rate to our interview requests and conducted interviews with 25 managers (20 men, five women). Of these, 23 were nationals of the programme country. Most had prior experience working with amphibians, although not necessarily in husbandry. Managers had backgrounds in various disciplines, including biology, zoology, taxonomy and veterinary science.
Assessment of programmes
Of the 23 programmes keeping amphibians, 10 were based in zoos, seven established by private individuals and two each based in universities, museums and research institutions. Programmes had highly diverse organizational structures. The smallest programmes consisted of one staff member, with a budget of a few thousand USD per year and keeping as few as 20 individuals of one species. The largest programmes had teams of over 10 staff, keeping up to 4,000 individuals of up to 16 species and with budgets between USD 100,000–1,000,000 per year. There was no association between the type of institution and the size of a programme or number of staff.
Five programmes were performing reintroductions and another four conducting release trials. The reintroduction programmes had budgets of USD 5,000–50,000, kept up to three species and employed up to three staff members. Three were independent programmes, one in a zoo and one in a university. The programmes had commenced in 2015, 2009, 2005, 2002 and 1991 (the latter ended successfully after 7 years). Three reported their releases had a positive impact on wild populations, and two were uncertain of the outcome. Of the three programmes with large budgets, two had not completed reintroductions, and one had performed one release trial. All three large programmes had operated for c. 10 years.
Programme partners
The four most important partners to programmes were zoos, the Amphibian Ark, government authorities and NGOs (Fig. 1a). Of the 13 partner zoos, eight were located out of country, five in the USA. Four of the seven partner NGOs were international. The most frequently provided resources by partners were (in rank order) funding, information and expertise, fieldwork and assistance, and training (Fig. 1b). Each partner commonly provided several resources.
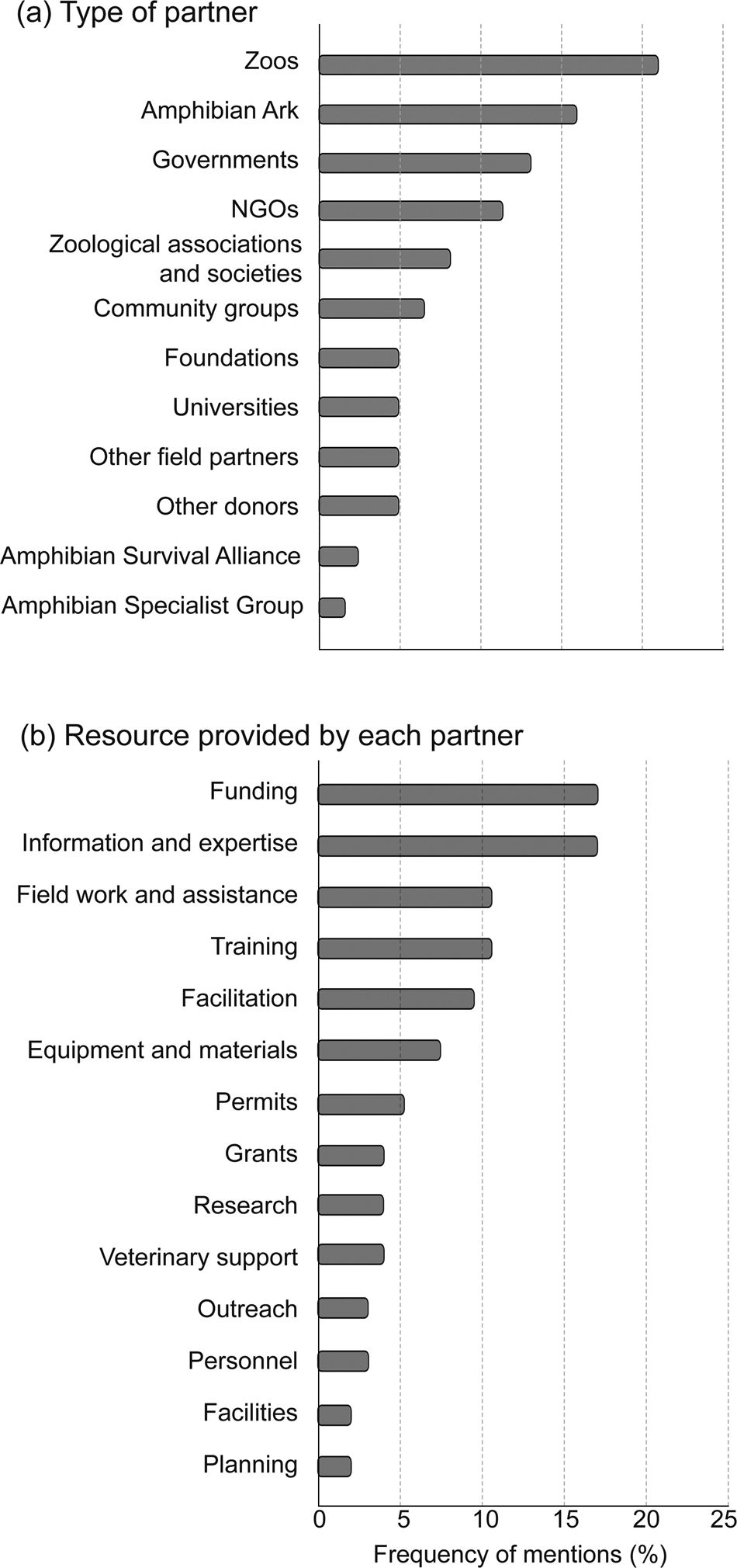
Fig. 1 (a) Partner types ranked by per cent of respondents identifying them as one of their three most important, and (b) resource types ranked by per cent of partners that provided them.
Barriers and enablers
During the thematic analysis, we identified 13 themes comprising 94 categories and 33 subcategories (Fig. 2; Supplementary Material 4). The number of times a category was mentioned did not necessarily indicate its significance. For example, only one manager mentioned lack of access to medicine to treat the programme's amphibians, but this was perceived as a critical barrier. Critical barriers were diverse (Fig. 3). Although material and financial resources were common barriers and enablers, human and institutional factors such as relationships and capacity were particularly common and often also critical. Four categories were both common and critical barriers and enablers: wild habitat conditions, public relations, government relations and captive environmental control systems. Despite some commonalities, most programmes faced unique barriers.

Fig. 2 The most critical and most common categories of barriers and enablers identified from the analytical framework, and the critical barriers summaries, based on the number of sources in which a category was identified.

Fig. 3 Summaries of the three most critical barriers for all recorded captive breeding programmes (each individual programme is represented by a box), their parent organization type (displayed by the border type of each box) and the stage in which the programme was at the time of the interview (circled on the left, and described under the operational model presented in Fig. 4).

Fig. 4 Key input required and support, positioned in line with the steps at which they occur (links with Fig. 3), illustrated for (a) a reactively implemented programme process on the top (currently most programmes), and (b) a proactively implemented programme process on the bottom (ideal process).
Operational model
A key theme that emerged was managers' desire to take a programme to the next stage in its development. Many managers had achieved a subset of their goals, but operational challenges, such as poor adaptability or a lack of specific resources, hindered progress towards reintroductions. Based on this finding, we developed a two-part operational model (sensu Knight et al., Reference Knight, Cowling and Campbell2006; Fig. 4), presenting a synthetic interpretation of commonly perceived barriers and enablers. Both models are simplifications of complex systems but include four key stages necessary for any captive breeding programme that aims to reintroduce individuals into the wild.
Firstly, we commonly observed a reactively implemented programme process (Fig. 4a), whereby decisions and actions responded to the perceived urgency of species declines. It presents a predictable pathway of barriers and enablers (sensu Linklater, Reference Linklater2003). Secondly, we developed a model of an ideal proactively implemented programme process (Fig. 4b), synthesized and structured from the barriers and enablers identified by managers. This model promotes strategic planning by providing recommendations for future implementation processes and partnership support.
Depending on the programme, overlaps and learning loops between the different stages can occur. Implementing the proactive model requires a shift in actions and resources throughout the programme process. We used this structure of stages to classify programmes, summarizing their respective critical barriers (Fig. 3).
Stage 1: Establishing a programme
This stage spanned the conceptualization of a programme to the collection of founder individuals. It typically involved the selection of species for which conservation action is most important and the preparation of facilities. Main barriers and enablers included availability of financial resources, infrastructure for captive breeding, species prioritization, captive environmental control systems, species-specific information, amphibian husbandry expertise, and staff training and expertise. Inadequate information on the threat status of species often hampered this stage, and poor planning and species prioritization often led to barriers at a later stage. This is illustrated by the statement of one respondent: ‘I think the biggest failure was the initial planning. Why? Because I would have liked to start with another programme, not just with what … is most attractive’.
A global network of partners was important as local capacity was often limited. Key partners were instrumental in facilitating a network, providing resources and access to interested donors. Parent organizations sometimes provided facilities and staff, and the Amphibian Ark seed grant often funded equipment. Partner input on staff training and facility design was important.
Stage 2: Husbandry and breeding
Husbandry and subsequent attempts to breed amphibians began after founder animals had been collected. The aim was to breed a sufficiently large, genetically viable founding population for the anticipated programme duration. This stage mainly comprised a learning process of developing husbandry and breeding protocols (Fig. 4). Main barriers and enablers were similar to Stage 1: captive environmental control systems, species-specific information, permits, food and nutrition, amphibian husbandry expertise, and staff training and expertise.
Many programmes managed to develop protocols despite lacking information on the target species' ecology, biology and requirements in captivity. Fifteen of 20 programmes were breeding viable offspring for one or more species, and only five programmes stated lack of breeding success for a species. Lack of staff training and expertise was a primary cause of failures: ‘they sprayed them with F10 [a disinfectant] thinking it was water… So again it's coming back to trained personnel being switched on’.
Programmes often lacked experience and knowledge on a species, and external advice was essential for resolving husbandry challenges. For independent programmes, limitations in fundraising for staff often led to low salaries and staff retention, whereas prioritization of staff time was a challenge in zoos. Staff training was the third most commonly provided resource by partners. Workshops, training courses and internships were undertaken, and informal discussions between managers and partners provided context-specific advice perceived as vital for problem solving.
Stage 3: Preparing for reintroductions
At this stage, managers worked proactively towards making reintroductions feasible, which included developing reintroduction protocols and mitigating threats in the wild. Barriers and enablers differed from previous stages and primarily included habitat conditions, government and public relations, bureaucracy, permits, allocation of resources for fieldwork and access to field sites.
The transition between Stages 2 and 3 was generally the most critical for programmes. Resources needed to expand beyond maintaining the captive collection to include fieldwork, monitoring habitats and populations, and investigating possibilities of threat mitigation. Failure to do so would result in stagnation. Partners were important in addressing limitations of time and resources: ‘We've got a bunch of existing monitoring work … and we recognize we just don't have the capacity to do this ourselves. And we're seeking additional partners who will be able to provide the manpower to come and take this project to the next level’.
The support required differed from previous stages, and included government support, funding, collaborations for fieldwork, research on habitat suitability, and technical reintroduction expertise.
Stage 4: Reintroduction and post-reintroduction
The release of captive populations into the wild and ongoing monitoring was affected by barriers and enablers including habitat conditions, government and public relations, and allocation of resources for fieldwork. Specialized tagging equipment was important for monitoring reintroduced populations. Ongoing monitoring, habitat management, stakeholder engagement and awareness raising were perceived as essential. External field partners were important contributors to these tasks: ‘The [captive] programme is finished but the government is still monitoring the release sites. And I mentioned one release site … the frogs disappeared after several years … But the management would like to bring them back again’.
Discussion
Amphibian captive breeding programmes are complex, dynamic systems presenting a high diversity of perceived, and often unique, suites of barriers and enablers. In our interviews with 25 programme managers, we found that effective implementation required alignment of many components. Failure could occur at any stage, and in numerous ways, often resulting from multiple factors, which is a common phenomenon documented in other fields (Helmreich, Reference Helmreich2000; Edmondson, Reference Edmondson2011; Hickey et al., Reference Hickey, Pham-Hung, Nosikova, Halvorsen, Gritti and Schwartz2017). Despite this complexity, we found common sets of barriers (Fig. 4a) that could be overcome with explicit strategic improvements (Fig. 4b).
Operational model
The literature provides numerous recommendations on how to plan captive breeding programmes (CMP, 2013; IUCN, 2013; Amphibian Ark, 2019). However, these recommendations are often not implemented, creating a typical knowing–doing gap (Pfeffer & Sutton, Reference Pfeffer and Sutton1999; Knight et al., Reference Knight, Cowling and Campbell2006, Reference Knight, Cowling, Rouget, Balmford, Lombard and Campbell2008). This is probably enhanced by the perceived urgency in the face of rapid rates of amphibian declines, which is at odds with the time required for planning and implementing effective programmes (Meredith et al., Reference Meredith, St. John, Collen, Black and Griffiths2017). Operational models are conceptualizations that guide the implementation of conservation, translating knowledge into action. They should promote stakeholder engagement, assessment, planning, management and social learning (Knight et al., Reference Knight, Cowling and Campbell2006). The operational model is based on self-reported information, meaning the values and cognitive biases of individual managers will have influenced decision-making.
The operational model was developed from lessons learnt based on successes and failures, the latter being an important but stigmatized and under-used tool for improving conservation practices (Catalano et al., Reference Catalano, Redford, Margoulis and Knight2018). The model structure recognizes that a lack of flexibility in resource allocation often hindered progress between stages. The reactive process represents managers' typical approach to planning reintroductions, and is valuable as a discussion tool to highlight shortcomings of current practices. The proactive process presents an ideal theory of change hypothesized to improve effectiveness. In combination, they can be used as a social learning tool. Our findings indicate that institutions can provide support beyond funding. However, restrictions wrought by the complexity and dynamism of social–ecological systems often limited implementation of an ideal model. We encourage zoos, NGOs and governments to use the operational model to identify ways in which they can support programmes, contributing to increased global capacity for safeguarding threatened amphibians.
Human dimensions
We documented several examples of restrictions caused by biological factors, such as the lack of breeding success until a species' reproductive behaviour in the wild was discovered, the difficulty of catering for a high-altitude species in a warmer, lower-altitude city, or lack of information and space to provide suitable nutrition. Although biological factors were evidently pivotal, human capacity was an equally important prerequisite. Human dimensions are overlooked in the captive breeding and conservation literature (Sutton, Reference Sutton2015; Catalano et al., Reference Catalano, Lyons-White, Mills and Knight2019). Decision-making is inevitably compromised by cognitive biases and other psychological and institutional phenomena. The so-called sunk cost fallacy, whereby perceived previous emotional and financial investments drive ongoing investment in a programme that is unlikely to succeed (Arkes & Blumer, Reference Arkes and Blumer1985), appeared present in some programmes.
Skilled, experienced staff were important at all stages, and lack of staff knowledge and expertise presented a major barrier for zoos in Western countries (Brady et al., Reference Brady, Young, Goetz and Dawson2017). Capacity building for amphibian conservation, however, is generally not highly prioritized by amphibian conservationists and practitioners (Meredith et al., Reference Meredith, St. John, Collen, Black and Griffiths2017). The value of leadership, open-mindedness, innovation and initiative were all identified in this study. Some managers spoke of the ability to achieve much with few resources when these could be allocated flexibly: ‘For me it's very difficult to get funds to pay people. I can get money to get equipment, or things like that … but not to pay people, and that is the main problem’.
Similarly, a manager's ability to change focus and balance tasks was essential in bridging the gap between Stage 2 (husbandry and breeding) and Stage 4 (reintroduction and post-reintroduction).
Support through strategic partnerships
Partners should aim to strategically develop collaborations to secure the diversity of resources required. Institutions and individuals can provide expert input at all stages, helping programmes overcome barriers and progress towards their goals. For example, the Amphibian Ark provides substantial support through seed grants and technical advice (Zippel et al., Reference Zippel, Johnson, Gagliardo, Gibson, McFadden and Browne2011).
Planning and prioritization
Careful planning, monitoring and evaluation should be an integral component from the beginning to identify where strategic support will be required over time. This will often involve prioritizing species to optimize cost-effectiveness of scarce resources (Bottrill et al., Reference Bottrill, Joseph, Carwardine, Bode, Cook and Game2008). Species prioritization has been the subject of much recent research and debate (e.g. Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015; Canessa, Reference Canessa2017; Griffiths, Reference Griffiths2017). Effective techniques are challenged by the increasing number of species requiring captive breeding programmes (e.g. Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015, Reference Tapley, Michaels, Johnson and Field2017; Griffiths, Reference Griffiths2017). We refer to species selected for the purpose of reintroduction, and acknowledge the many contributions of programmes beyond reintroductions. Species prioritization techniques should be reviewed as new information emerges (Griffiths, Reference Griffiths2017), and grounded in an evidence-based process; which is what Amphibian Ark's Conservation Needs Assessment aims to help achieve (Johnson et al., Reference Johnson, Baker, Buley, Carrillo, Gibson and Gillespie2018). The risk of pathogen transmission should also be considered as it poses a significant threat to captive and wild populations, which increases when species are kept outside their geographical range and/or in cosmopolitan collections of multiple species from different geographical locations (Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015). Cosmopolitan collections pose a quandary for many international zoos wishing to assist conservation efforts by increasing holdings of threatened amphibians (Griffiths, Reference Griffiths2017; Tapley et al., Reference Tapley, Michaels, Johnson and Field2017).
A clear understanding of ultimate goals, and how and when a programme should be terminated, are crucial planning elements. Many programmes suffer from poor implementation of species management plans, studbooks and exit strategies, which hinders effectiveness (K. Johnson, pers. comm., 2018). There are numerous tools available for planning conservation programmes, such as guidelines for developing a species action and recovery plan (Amphibian Ark, 2019), reintroduction and translocation guidelines (IUCN, 2013), and the Open Standards for the Practice of Conservation (CMP, 2013). The operational model presented here can assist managers with the implementation of planning tools.
Effective operational models explicitly identify how progress will be monitored and evaluated (Knight et al., Reference Knight, Cowling and Campbell2006; CMP, 2013), however, only one manager mentioned these activities. External and internal evaluation processes urgently require wider recognition and improvement (Fisher et al., Reference Fisher, Balmford, Ferraro, Glew, Mascia, Naidoo and Ricketts2014). Internal evaluations are the foundation for individual and team learning (Catalano et al., Reference Catalano, Lyons-White, Mills and Knight2019), and external evaluations have the ability to question a programme's focus and improve performance (Kleiman et al., Reference Kleiman, Reading, Miller, Clark, Scott and Robinson2000). Partners could provide significant support in this regard.
Programmes should establish clear indicators and targets for monitoring and evaluation to enable decisions on where to proceed. Effective operational models and plans are dynamic and complex rather than linear, with learning loops feeding adaptive processes through monitoring and evaluation (Knight et al., Reference Knight, Cowling and Campbell2006; CMP, 2013). Indicators and targets are required to reduce the impacts of genetic inbreeding and adaption to captivity, especially for longer-term programmes (Robert, Reference Robert2009; Tapley et al., Reference Tapley, Bradfield, Michaels and Bungard2015). A failure to align processes and outcomes can result in programmes not achieving desired outcomes (Kleiman et al., Reference Kleiman, Reading, Miller, Clark, Scott and Robinson2000; Meredith et al., Reference Meredith, St. John, Collen, Black and Griffiths2017). Many programmes are currently unlikely to reach the reintroduction stage (Griffiths & Pavajeau, Reference Griffiths and Pavajeau2008; Harding et al., Reference Harding, Griffiths and Pavajeau2016), emphasizing the importance of monitoring and evaluation to improve cost-efficiency (Bottrill et al., Reference Bottrill, Hockings and Possingham2011; CMP, 2013), and to avoid the sunk cost fallacy trap.
Links to in situ conservation
Captive breeding programmes should complement in situ conservation (Pritchard et al., Reference Pritchard, Fa, Oldfield and Harrop2012; Byers et al., Reference Byers, Lees, Wilcken and Schwitzer2013). However, we found that programmes often lacked links to in situ partners. Harding et al. (Reference Harding, Griffiths and Pavajeau2016) similarly found that only 64% of programmes had links to in situ conservation initiatives. Establishing links to develop an integrated field component was not always perceived as feasible because of a lack of resources or experience. Paradoxically, this contradicts the perception by amphibian conservationists that species and habitat improvements are the most important foundations for programme success (Meredith et al., Reference Meredith, St. John, Collen, Black and Griffiths2017). Most programmes with an in-situ conservation component were located within the species' native range in-country, whereas other activities such as monitoring amphibian populations and engaging in awareness raising and education were sometimes undertaken outside the captive species’ native range or country, and these activities were slightly favoured by larger, multi-species programmes.
Ensuring captive breeding programmes are linked with field programmes is an action within the Amphibian Conservation Action Plan (Wren et al., Reference Wren, Angulo, Meredith, Kielgast, Dos Santos and Bishop2015). Broadening the network of field conservation partners to more effectively link ex situ and in situ mechanisms could facilitate this. The One Plan Approach (a well-planned, collaborative, integrated programme design in which programmes complement in situ conservation; Byers et al., Reference Byers, Lees, Wilcken and Schwitzer2013) encourages zoos to support such linkages through provision of grants, internship opportunities for field components of programmes, and advocating for increased institutional and government support.
Collecting and sharing information
Programmes and zoos have identified a lack of information, knowledge and expertise as a barrier to success (Brady et al., Reference Brady, Young, Goetz and Dawson2017). Research on a species' threat status and habitat requirements should precede the establishment of a programme (Michaels et al., Reference Michaels, Gini and Preziosi2014), as this will support an evidence-based approach and allow for valuable knowledge exchange through social learning; a prerequisite for successfully navigating the transition from research to implementation (Toomey et al., Reference Toomey, Knight and Barlow2017). Social learning institutions provide expertise for adapting management activities, and should complement the implementation of operational models (Knight et al., Reference Knight, Cowling and Campbell2006, Reference Knight, Cowling, Rouget, Balmford, Lombard and Campbell2008). The Amphibian Ark acts as such an institution. However, this expertise is currently mainly accessible through pre-established partnerships, a husbandry documents library and a newsletter (Amphibian Ark, 2019). Social media groups could provide a platform for expert networking, driving timely problem solving, and the ethical involvement of students and interns for targeted research on poorly known species should be facilitated when possible. In addition, programmes must embrace the urgent need to improve implementation of future programmes by disseminating lessons learnt from past failures (Catalano et al., Reference Catalano, Lyons-White, Mills and Knight2019).
Limitations and future research
Linguistic barriers and lack of prior experience with amphibian captive breeding programmes potentially influenced the lead researcher's interpretation of the data. Further research into the human dimensions of implementing effective programmes will be essential to confirm, or not, the findings presented here.
It is possible that people who initiate amphibian captive breeding programmes, feeling attachment and responsibility towards their target species, are not capable of recognizing a lack of progress as a failure because of confirmation bias (Catalano et al., Reference Catalano, Redford, Margoulis and Knight2018). As a result, programmes might stagnate when they would benefit from a strategic change. Further research is required to examine how the psychology and dynamics of individuals and teams influence their operations and effectiveness (Catalano et al., Reference Catalano, Redford, Margoulis and Knight2018).
Partnerships have been identified as critical to effective programmes. Zoos were the main partners, contributing through a wide variety of activities. These contributions are reasonably well understood as they are periodically reviewed. Further research into the contributions of international NGOs is required to target captive breeding initiatives more strategically. This could be complemented with research into how programmes can increase support for targeted field components, improving the availability of suitable habitats for both captive and wild populations, thereby assisting managers to navigate the transition between ex situ and in situ conservation more effectively.
The barriers and enablers identified in this study are probably shared by captive breeding programmes for other taxa. Future research could investigate the similarities and differences between taxa as a basis for adaptation of the operational model for other species. Explicit testing of these operational models could enhance the effectiveness of species-specific conservation programmes.
The operational model, in combination with other available planning tools, provides an opportunity for a more collective, systematic and strategic approach to amphibian captive breeding programmes. This has the potential to increase the effectiveness of these programmes and thus improve outcomes for amphibian conservation.
Acknowledgements
We thank L. Carrillo, J. Shu, J. Perez, K. Baatrup and N. Barker for their input in conducting interviews, translating transcripts and developing the analytical framework; and the interviewees who took time out of their busy schedules to participate in this study.
Author contributions
Study design: all authors; data collection and analysis: BK; writing: all authors.
Conflicts of interest
This work was funded by Imperial College London, with no conflict of interest. The work was conducted in partnership with Durrell Wildlife Conservation Trust and the Amphibian Ark. These organizations have relationships with most of the interviewees, and although overwhelmingly positive, had the potential to bias responses. The observational standpoint of the lead interviewer (BK) has been documented (Supplementary Material 3b).
Ethical standards
Ethics approval was provided by Imperial College London, and the work otherwise abided by the Oryx guidelines on ethical standards. Managers were provided a short introduction on the research topic and objectives, and asked to sign prior informed consent forms.









