INTRODUCTION
Respiratory diphtheria, usually caused by toxigenic strains of Corynebacterium diphtheriae, is a vaccine-preventable disease that can affect persons of all ages. Although the global incidence of diphtheria has decreased significantly in the last three decades, the disease remains a problem, especially amongst young children in countries with low immunization coverage [1].
In South Africa, diphtheria-containing vaccines are part of the Expanded Programme on Immunization (EPI) for children and are available at no cost in the public health sector. A primary series of three vaccinations with diphtheria toxoid are given at 6, 10, and 14 weeks of age administered as a combination vaccine. At 18 months, a booster dose is given using the same vaccine as for the primary series. Since 2008, two additional booster doses with a reduced concentration of toxoid are recommended at 6 and 12 years of age [2].
KwaZulu-Natal is one of nine provinces in South Africa and is divided into 11 health districts. In 2014, according to the data from the District Health Information System which is used to manage routinely collected health service-based information [3], the coverage of the primary series diphtheria vaccinations in the province was 96%, and the 18 month booster vaccination was administered to 83% of children at that age. The coverage for the 6 year and 12 year booster vaccinations was 54% and 20%, respectively.
Since 2000, only seven cases of diphtheria have been reported in South Africa, the last of which was documented in 2010 [4]. In KwaZulu-Natal, the last reported diphtheria case was in 1989 [Reference Jeena, Wesley and Coovadia5].
Between March and June 2015, 15 cases of diphtheria were identified in two health districts in the KwaZulu-Natal Province in South Africa. We describe the clinical, laboratory, and public health aspects of this outbreak.
METHODS
Case definitions
After the first case was diagnosed, case definitions were drawn up as part of management guidelines to facilitate case finding. Cases were classified as suspect, probable, or confirmed (Table 1).
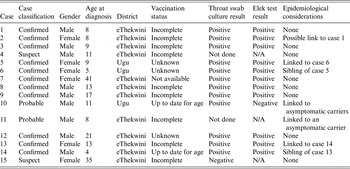
Table 1. Case definitions implemented during the diphtheria outbreak in KwaZulu-Natal, 2015

Laboratory diagnosis
Throat swabs from suspected cases were plated on 5% horse blood agar and Hoyle's media (Diagnostic Media Products, National Health Laboratory Service, Johannesburg, South Africa) with an additional chocolate agar plate for tissue specimens. Nasal swabs from contacts and nasopharyngeal swabs collected during the enhanced surveillance were plated onto Hoyle's agar only. Plates were incubated for 48 h. All Gram-positive bacilli that grew as black pigmented colonies on Hoyle's plates were identified using biochemical tests and Matrix-Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF) (bioMérieux S.A.). Toxin production and the presence/absence of the A and B subunits of the C. diphtheriae toxin (tox) gene were confirmed by the Elek test and real-time PCR, respectively [Reference Efstratiou6, Reference Mothershed7]. Multilocus sequence typing (MLST) was used to characterise the isolates [Reference Bolt8]. The sequence types (STs) were extracted from whole genome data and compared with all available STs (n = 437) listed in the global MLST database (http://pubmlst.org/cdiphtheriae/).
Co-ordinated outbreak response
A multidisciplinary provincial outbreak response team was established following identification and confirmation of the first case of diphtheria. The team included senior district and municipal health management, communicable disease control (CDC) managers and co-ordinators, clinicians, microbiologists, an epidemiologist, and pharmacy managers. In addition the following departments were represented: school health and EPI, occupational health, health promotion, environmental health, infection prevention and control, and public health medicine. The team met weekly, discussed cases, presented findings and activities of field teams and agreed upon response measures. The provincial CDC unit was responsible for the co-ordination of outbreak response activities, and the district outbreak response teams were responsible for the implementation of these activities.
The National Institute for Communicable Diseases (NICD) Outbreak Response Unit provided support to through the compilation of case definitions and management guidelines, collation of epidemiological data on each case, and provision of a daily situational report. The NICD operated a 24-h ‘hotline’ to assist with case identification, management, and reporting.
Case detection
The district outbreak response teams alerted staff at public and private healthcare facilities to be aware of the possibility of diphtheria. Media reports facilitated increased public awareness. Community caregivers (non-professional individuals providing care within communities) were trained on the signs and symptoms of diphtheria.
Clinical management and case investigation
A clinical protocol on the diagnostic criteria and acute management of suspected diphtheria cases was distributed to all healthcare facilities in the province. Each suspected case was discussed with a provincial paediatric infectious disease specialist (M.A.) who evaluated the patient's clinical presentation, and assessed the need to administer anti-toxin. Suspected cases were managed at the hospital where they presented. Suspected cases were treated for 14 days with either intravenous soluble penicillin (50 000 IU/kg), or azithromycin (10 mg/kg). Patients who required ventilation were transferred to a tertiary-level hospital with intensive care facilities. When a suspected case was identified, a case investigation form was completed with demographic and medical history, clinical management and outcome. Vaccination status was ascertained by review of Road-to-Health card. If this card was not available, vaccination status was reported as ‘unknown’.
Diphtheria antitoxin (DAT) was first purchased from India, and subsequently donated by the Republic of Japan (Vaccine Business Section, Sales Division, Kaketsuken, Chemo-Sero-Therapeutic Research Institute), but this was not available when the first three cases were identified.
Contact tracing
Following identification of a probable case, and before laboratory confirmation, contact tracing was initiated by a local area-based outbreak response team led by CDC co-ordinators. A contact was defined as someone who had direct physical contact with a case, or who either lived in the same household as the case or spent a significant amount of time with the case, for example learners sharing the same classroom or work colleagues sharing the same office. The CDC co-ordinator, together with the infection control practitioner at the healthcare facility identified any healthcare worker that was involved in the management of cases, including paramedical staff who transported cases between healthcare facilities. Nasal swabs were obtained from contacts. A ‘contact investigation form’ was completed for all contacts to obtain demographic details, and their clinical history and details of all possible contacts.
Enhanced surveillance
Enhanced surveillance was done in five primary healthcare clinics, in the areas of the eThekwini Health District from which the majority of cases emanated. This surveillance commenced in June 2015 and aimed to identify cases of diphtheria of mild-to-moderate severity over a 6-week period. Trained nurses obtained nasopharyngeal swabs from adults and children with symptoms of an upper respiratory tract infection (cough, fever, runny nose).
Targeted vaccination
A targeted vaccination campaign was commenced at schools closest to the areas from which the cases emanated. Learners aged 6–15 years were targeted to receive a booster dose of diphtheria vaccine (given in combination with tetanus as Diftavax®). Learners were provided with consent forms, and those learners who obtained signed parental consent were vaccinated.
Ethics
Permission was obtained from the KwaZulu-Natal Department of Health Research and Ethics Committee to publish patient data. The NICD has ethics approval from the Human Research Ethics Committee of the University of the Witwatersrand to publish data from outbreak investigations (M160667).
RESULTS
Index case
On 15 March 2015, an 8-year-old male presented to a regional hospital in eThekwini Health District in respiratory distress with neck swelling, drooling, and difficulty swallowing. On examination, he had massively enlarged tonsils with a pseudo-membrane over the tonsils and soft palate. He was transferred to a tertiary hospital where he underwent a tracheostomy. The otorhinolaryngologist (W.K.) who attended to the patient made a clinical diagnosis of diphtheria. Endotracheal swabs taken yielded C. diphtheriae. The patient spent 4 days in intensive care and was transferred to the referring hospital following clinical improvement. The confirmation of the diagnosis in this patient led to the activation of the district, provincial and national outbreak response structures as described above. At the time, DAT was not available in the country.
Descriptive epidemiology
After the first case, there were three more cases in eThekwini Health District over the ensuing 2 weeks. Four weeks later, the first case was reported from Ugu Health District which is approximately 120 km away from eThekwini Health District (Fig. 1). The last case was reported on 12 June 2015. In total there were 15 cases over a 14-week period (Fig. 2). Twelve cases were from eThekwini Health District and three cases from Ugu Health District. There was no travel history amongst the cases or contacts interviewed.

Fig. 1. Geographic location of 15 diphtheria cases identified during an outbreak in KwaZulu-Natal, 2015.

Fig. 2. Number of diphtheria cases by week of illness and district, KwaZulu-Natal, 2015.
The age of the patients ranged from 4 to 41 years with a median age of 10. The majority (n = 7, 47%) were in the age group 6–11 years. Nine of the cases (60%) were male. Of those who were <18-years old (n = 12), the majority (n = 9, 75%) were incompletely or never immunized. Two of the cases did not have immunization records, and were classified as unknown (Table 2).
Table 2. Demographic characteristics and laboratory results of 15 diphtheria cases identified during an outbreak in KwaZulu-Natal, 2015
The overall CFR (case-fatality ratio) was 27% (n = 4), with all deaths occurring in eThekwini Health District.
Clinical presentation and management
The mean time from onset of symptoms to admission was 3 days (range 1–5 days). The commonest clinical sign on presentation was tonsillitis/pharyngitis (n = 14, 93%) followed by fever (n = 11, 73%). Visualisation of a pseudo-membrane and the presence of a bull-neck was reported in 10 cases (67%). Twelve of the 15 cases (80%) presented with severe respiratory distress requiring acute airway management or monitoring. The two cases who presented with mild respiratory symptoms were fully immunized. Thirteen of the cases were HIV uninfected (Table 3).
Table 3. Clinical features and management of 15 diphtheria cases identified during an outbreak in KwaZulu-Natal, 2015
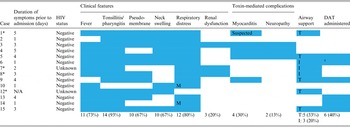
M, Mild disease; I, intubated; T, tracheostomy. *Patient demised.
† Delayed administration of DAT.
Two patients required surgical airway management. Four additional cases required ventilation.
Myocarditis was diagnosed in four patients (27%). This diagnosis was based on clinical, electrocardiogram and echocardiographic features. Myocarditis developed on average 14 days after admission to hospital and all 14 patients recovered without sequelae following medical management. Two of the four patients received DAT; 12 days and 4 days post-admission, respectively. An additional patient had a sudden unexpected death during the convalescence phase of the infection which was suspected to be secondary to an undiagnosed myocarditis. No post-mortem was done to confirm this.
Three patients developed renal dysfunction. Two of these patients recovered with simple fluid therapy; one of whom had received DAT. One patient died from multi-organ failure with renal failure playing a major role.
Two patients developed a peripheral neuropathy, both patients presenting with a pseudo-bulbar palsy 4 weeks after the initial admission. Neither patient received DAT.
A further two deaths occurred; one was attributed to systemic-related toxicity and the other demised at home before presentation to a healthcare facility.
Laboratory results
Specimens for culture were received from 13 of the 15 patients. The two cases without laboratory specimens included the case that demised at home and a case that was managed in a private hospital. Toxin production was confirmed in all isolates that carried the tox gene. Eleven of the 13 isolates were toxin positive and were classified as confirmed cases. Two of the cases with toxin-negative isolates were treated as probable cases as they were linked to asymptomatic carriers. One case-patient yielded two isolates: a toxin-positive isolate and a toxin-negative isolate.
An additional six toxin-positive isolates were from asymptomatic carriers epidemiologically linked to cases. This was carefully reviewed to exclude laboratory error. Two additional toxin-negative C. diphtheriae isolates were found: one was a contact of a case, and one was from a patient with possible cutaneous diphtheria.
Two novel STs were identified among the outbreak isolates, none of which were related to any other STs listed in the global PubMLST database at the time of this analysis (http://pubmlst.org/cdiphtheriae/). All 17 toxin-positive isolates collected from cases and contacts during the outbreak were ST-378. The toxin-negative isolates were ST-395.
Outbreak response
Contact tracing
Ninety-three household contacts, 981 school or work contacts and 595 healthcare workers were identified. All of the healthcare workers received prophylactic antibiotics (azithromycin or erythromycin, depending on availability) and the diphtheria booster vaccine. All household contacts received prophylaxis and 88 (95%) received the diphtheria booster vaccine. Two contacts (siblings of cases 5 and 13) progressed to developing diphtheria symptoms after receiving prophylaxis and were classified and managed as cases. There were no other cases emanating from the households, work places or schools at which contacts received prophylaxis.
Targeted vaccination
There are 805 schools in the eThekwini Health District with a population of 411 977 in the targeted age group. Only 446 (55%) of these schools were visited during the outbreak response resulting in a 52% immunization coverage. In Ugu Health District, there were 209 schools with a target population of 77 406. One hundred and eighty (86%) schools were visited and 39% of the target population was vaccinated. The targeted vaccination campaign occurred in May and June, during epidemiological weeks 21–25. During the campaign, the four cases (cases 11–14) that were identified were not from the schools, or communities near the schools, that had been visited as part of the campaign. There were no further cases reported after the targeted vaccination campaign ended.
Community participation and mobilization
A total of 195 community care givers were trained on the signs and symptoms of diphtheria in the two affected districts.
Surveillance
Nasopharyngeal swabs were obtained from a total of 782 adults and children with symptoms of an upper respiratory tract infection. None of these specimens tested positive for C. diphtheriae.
DISCUSSION
The majority (75%) of the cases in this outbreak of diphtheria were incompletely immunized and the outbreak occurred in a population with low immunization coverage of the 6- and 12-year booster vaccinations. The predominant populations affected in diphtheria outbreaks are children and adults with waning immunity [Reference Galazka and Dittmann9]. In recent diphtheria outbreaks in Nigeria and Brazil, the majority of the cases were partially immunized or unimmunized – 98% and 62%, respectively [Reference Besa10, Reference Santos11]. KwaZulu-Natal has a high burden of childhood mortality, mainly from diarrhea and pneumonia [Reference Massyn, Padarat, Barron and Day12]; and diseases that do not contribute to this high morbidity and mortality are often neglected. Prior to this outbreak, the low coverage of the diphtheria booster vaccinations in all districts in the province had not been enhanced through catch-up campaigns. In the absence of programmatic booster vaccinations after childhood, waning immunity increases the susceptibility of older children as well as adults [Reference Mattos-Guaraldi13].
In countries with high vaccination coverage, it is postulated that diphtheria can occur as a result of the importation of new strains of toxigenic C. diphtheriae [Reference Galazka and Dittmann9]. This assumes a variation in antigenic structure of the toxin for which vaccination does not offer protection. Although the novel ST (378) of the isolates makes importation of C. diphtheriae unlikely; it may have been imported from a country with poor microbiological diphtheria surveillance. However, the lack of travel history amongst cases and contacts decreases this likelihood. The outbreak was restricted to only two districts in the province which are approximately 120 km apart. It is probable that there was unreported travel history amongst the cases or controls that linked these two districts. It is unclear why there were no cases from the surrounding districts especially since eThekwini Health District is the most populous district in the province with a wide transport network. The outbreak occurred concurrently with the influenza season in KwaZulu-Natal, and mild cases of diphtheria may have been misdiagnosed. However, the enhanced surveillance at clinics in eThekwini Health District yielded no positive C. diphtheriae isolates.
Crowding and poor hygiene can facilitate disease transmission [Reference Quick14]. Although many of the cases emanated from areas with poor socio-economic conditions, we did not observe a high transmission rate of the disease. The epidemic curve also highlights the sporadic nature of the outbreak. Cutaneous diphtheria is known to be a reservoir of circulating strains which may become toxigenic [Reference Bowler15, Reference Hart16]. Nevertheless, this was not the case during the KZN outbreak as the non-toxigenic isolates belonged to an unrelated genotype.
Underlying immunosuppression did not appear to be a contributor to the transmission or manifestation of diphtheria amongst the cases. Although the outbreak occurred in an area with a high HIV prevalence, none of the patients were known to be HIV infected. The 27% case fatality in this outbreak is higher than other recent outbreaks reported in Nigeria (21%) and India (20%) [Reference Besa10, Reference Das17]. Only one of the cases demised prior to the availability of DAT. One of the deaths may have been linked to poor access to healthcare services.
Contact tracing was successfully conducted for all cases. Vaccination coverage of 100% was not achieved among contacts because in the initial phase of the outbreak, it was not clearly communicated that all contacts, irrespective of age, should receive a diphtheria booster vaccination. The high number of healthcare workers that were identified as contacts is disproportionate to the number of cases during the outbreak. In some areas, healthcare workers were concerned about the possibility of acquiring the disease and identified themselves as contacts even though they were not likely to have been in contact with a diphtheria case. Whilst it would have been ideal to offer all healthcare workers a booster dose of diphtheria vaccine, there was insufficient stock of the vaccine to do so. The nasal swabs taken during contact tracing were not ideal specimens as nasopharyngeal specimens are recommended for identifying asymptomatic carriers. The correct flexible swabs are not routinely available in our healthcare setting.
We were unable to extend the immunization to all learners that would have been eligible to receive a diphtheria booster vaccination (anyone who missed a booster dose) due to insufficient vaccine stock. Although a large proportion of schools were reached as part of the targeted vaccination drive, there were challenges encountered. First, the school health teams had difficulty negotiating time to vaccinate learners as the schools had commenced with mid-year examinations. Second, many learners did not bring back signed consent forms and in some instances learners older than 12 years did not assent to being vaccinated. Despite these limitations, the vaccination campaign is likely to have contributed to the halt in transmission of diphtheria in the affected districts.
CONCLUSION
Maintaining high coverage of diphtheria booster vaccines in adolescence is vital to prevent outbreaks of this disease. There is an urgent need to improve this immunization coverage in KwaZulu-Natal. School vaccination programmes should be ongoing until all districts in the province have achieved an immunization coverage of at least 80%. At a national level, the feasibility of offering all teenagers and adults a diphtheria vaccine booster if they have not been immunized against diphtheria in the previous 10 years should be considered. Ten-yearly diphtheria booster vaccination should be included in the routine package of services offered to all healthcare workers. In areas with sub-optimal diphtheria vaccine coverage, a stock of DAT should be readily available to facilitate optimal management of diphtheria cases.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the eThekwini and Ugu District CDC co-ordinators (Mr Mbuthu, Ms Ndlovu, Mr Mhlongo, Mr M Hadebe and Ms Mlambo) and their teams, Ms Gumede and Ms Fynn, Ms Dladla, Dr Z Banoo, the school health teams, Samuel Candice; and all other members of the provincial and district outbreak response teams for their role in managing the outbreak. They would also like to acknowledge laboratory support from Valentino Horne, Diagnostic Media Production Green Point, National Health Laboratory Service, Cape Town; staff at the Centre for Respiratory Diseases and Meningitis, NICD, Johannesburg; and the technical assistance from the World Health Organization.
DECLARATION OF INTEREST
None.










