Introduction
Tuberculosis (TB) in children has received increasing attention in recent years, with the World Health Organization (WHO) being particularly interested in improving the estimates of the burden of child morbidity and mortality from TB and its contribution to the global burden of TB [Reference Graham1–4]. It is widely recognised that infants and young children (<5 years of age) are at high risk of progression to active disease following infection with Mycobacterium tuberculosis and are particularly prone to severe, disseminated forms of TB [Reference Marais5, Reference Seddon and Shingadia6]. While the risk of active disease is much lower in the 5–9-year-old age group, early epidemiological observations in the pre-human immunodeficiency virus (HIV) era reported a sharp increase in risk again through the adolescent and young adult years (ages 10–24) [Reference Marais5, Reference Comstock, Livesay and Woolpert7, Reference Morabia8]. As adolescents and particularly young adults who develop TB often present with ‘adult-type’ pulmonary TB that is sputum smear-positive [Reference Marais5], they are far more likely than younger children to contribute to on-going transmission in the community. Furthermore, evidence is emerging that adolescents and young adults (‘young people’) with TB may have complex adherence support needs [Reference Isaakidis9], and may be at elevated risk of treatment discontinuation relative to both children and to adults [Reference Enane10, Reference Snow11]. As such, young people (aged 10–24) arguably constitute an important but neglected group within global TB control.
It is increasingly appreciated that the adolescent and young adult years are a time of rapidly changing burden of disease, which presents new opportunities for public health interventions [Reference Gore12, Reference Sawyer13]. The International Roadmap for Childhood TB explicitly called for the attention of the needs of adolescents at risk of TB in research, policy development and clinical practices, with the first step of a suggested framework being to ‘know your epidemic’ [2]. With regard to TB among adolescents and young adults, this is challenging for a number of reasons. At present, data on TB burden are routinely reported by national TB programmes to the WHO disaggregated into broad age groups (5–14, 15–24 years) which complicates burden of disease estimation among young people [3]. A recent analysis of global TB surveillance data suggests that between 1.2 and 3.0 million adolescents and young adults develop TB each year, with the considerable uncertainty around the estimates due to limitations in available data [Reference Snow14]. Adolescent-specific data are rarely reported in publications, despite the presence and adolescents and young adults in many clinical cohorts. Similar challenges pervade reporting on other communicable diseases such as HIV, obscuring our understanding of the major causes of morbidity and mortality in adolescents and young adults as a distinct group [Reference Idele15].
TB among young people is epidemiologically complex and may require specialised health system responses with regards to prevention and management, but currently, even the most basic epidemiological information regarding the burden of TB in adolescence and young adulthood – estimates of incidence and prevalence – are lacking. The primary aim of this systematic review was to identify direct estimates of the incidence and prevalence of bacteriologically confirmed pulmonary TB among adolescents and young adults aged 10–24 years, as measured using active case finding approaches.
Methods
We searched PubMed with the following string on 14 June 2014 and again on 20 August 2017: (‘tuberculosis‘[Title]) AND (‘adolescent‘[MeSH Terms] OR ‘adolescent‘[All Fields] OR ‘adolescents‘[All Fields]) AND (‘epidemiology‘[Subheading] OR ‘epidemiology‘[All Fields] OR ‘incidence‘[All Fields] OR ‘incidence‘[MeSH Terms] OR ‘prevalence‘[All Fields] OR ‘prevalence‘[MeSH Terms]). We also hand-searched archives maintained by the WHO's Global TB Programme for written reports and unpublished data on national prevalence surveys supported by the WHO global task force on TB impact measurement [Reference Floyd16].
The review questions and search strategy were decided a priori. KS and LN independently screened the PubMed search results and identified studies for full-text retrieval. Disagreements were resolved through discussion with CS. KS retrieved the full texts, confirmed eligibility and extracted the data. We contacted authors of prospective studies and national prevalence surveys that included adolescents but did not report adolescent-specific estimates to request additional data.
Inclusion criteria
Inclusion and exclusion criteria were defined so as to limit the risk of bias due to unrepresentative sampling or diagnostic criteria with low-specificity. Studies were eligible for inclusion if they:
• Reported data for an observation period that began during or after January 2000;
• Reported age-specific observations of incidence or prevalence in an age group with a minimum lower bound of 10 years and a maximum upper bound of 24 years (e.g. 12–17 years, 15–24 years);
• Used at least one of sputum smear microscopy, culture or the Xpert MTB/RIF® assay to confirm suspected TB;
• Included at least 100 participants in an age group of interest, drawn from a representative sample of the source population.
Exclusion criteria
• Publications reporting exclusively on passively collected surveillance data were excluded. National surveillance data are summarised in WHO's global TB database, which is publicly available online [17]. This review was restricted to primary research data collected through active case finding, to address the issues of underdiagnosis and underreporting which can occur through passive case detection and surveillance.
• Studies reporting on broad age groups that included adolescents or young adults but did not differentiate them from younger children or older adults (e.g. 10–35 years) were not included.
• Studies reporting exclusively on non-representative population sub-groups (e.g. remote Indigenous communities, migrants, prisoners) were excluded unless the study reported on a sample of young people living with HIV.
Data analysis
Age-specific incidence and prevalence estimates were extracted from each eligible paper. Where possible, missing parameters (e.g. 95% confidence intervals) were calculated from the data presented using Stata 13 (StataCorp, Texas, USA).
National estimates of TB caseload in the relevant age groups were derived from national prevalence survey data and United Nations Population Division estimates for the relevant age groups in the nearest calendar year to the survey (2000, 2005, 2010 or 2015), where estimated TB caseload = TB prevalence per 100 000 * (population in age group/100 000). National estimates were not calculated from sub-national studies, as studies conducted in subnational areas cannot be assumed to represent entire nations. No meta-analysis was performed as the resulting pooled estimate would be determined by the settings of the included studies and would not represent the average value worldwide.
Risk of bias within studies was assessed using a customised checklist assessing the sampling strategy, screening algorithm, diagnostic criteria and statistical adjustment for clustering and missing data within the age bracket. Risk of bias across studies was assessed with regard to both geographic representativeness and the epidemiological characteristics of the included studies in aggregate.
Results
Search results
Our initial PubMed search returned 3540 results, of which 449 full texts were screened and 12 were found eligible for inclusion. Studies rejected at the title review stage were most often rejected due to ineligible study designs (e.g. case series, reports on routine surveillance data). Studies rejected after abstract or full-text screening were most often rejected because they did not include young people, because they were not population-based studies (e.g. surveys of army recruits) or because they did not present age-specific data for an appropriate age group (see Fig. 1). The updated search identified 475 results, of which 12 full texts were screened and four found eligible for inclusion. An additional 11 publications or reports were identified in WHO records.

Fig. 1. PRISMA flowchart of study selection process.
Among the 27 included publications only two pertained to prospective studies; the remainder reported the results of cross-sectional prevalence surveys. Two prevalence surveys resulted in two publications each, as did one cohort study, resulting in a total of 27 publications from 24 studies. Data from a further two national prevalence surveys were obtained prior to the publication of their reports.
No epidemiological studies reporting laboratory-confirmed TB in adolescents or young people living with HIV were identified. A small number of clinical cohort studies pertaining to children engaged in clinical care for HIV were screened. None of these studies included adolescent-specific data, and all used a clinical case definition of TB without laboratory confirmation, highlighting the persistent challenges with diagnostic confirmation in this group.
Of studies excluded after review of the full text, 68 had included adolescents in the study but did not present TB incidence or prevalence disaggregated by age group. Among the authors who responded to our requests for disaggregated data, one was able to provide case numbers of bacteriologically confirmed TB in adolescents in South Africa [Reference Marais18]. Additional age-disaggregated data were obtained from WHO records on national prevalence surveys in China [Reference Wang19], Nigeria [20], Ethiopia [21, Reference Kebede22], Cambodia [23], Pakistan [Reference Qadeer24, Reference Qadeer25], Ghana [Reference Bonsu26], Thailand [27], the Philippines [28], Bangladesh (report in preparation) and Kenya (report in preparation).
Of the 30 countries that WHO defines as high TB burden [29], 15 were represented in at least one of the identified studies. In several of the remaining 15 countries, WHO-supported national TB prevalence surveys are either completed, underway or planned [30].
Study results
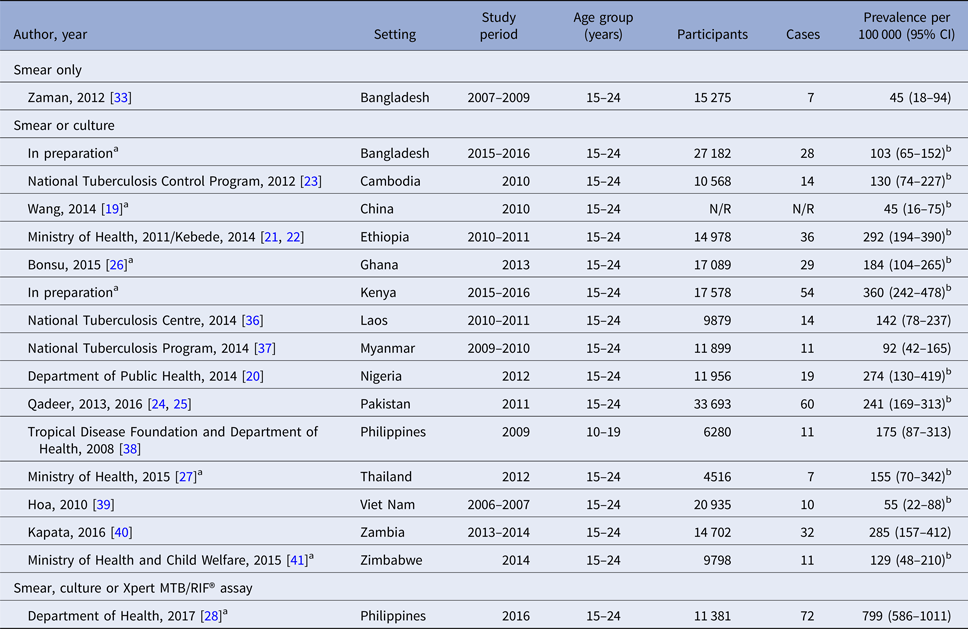
Prevalence estimates from studies using both smear and culture ranged from 45 per 100 000 in China [Reference Wang19] to 241 per 100 000 in Pakistan [Reference Qadeer24] in the Asia-Pacific region, and from 160 per 100 000 in Basuoga, Uganda [Reference Waako31] to 462 per 100 000 in Lusaka, Zambia in sub-Saharan Africa [Reference Ayles32]. Two studies from Bangladesh using smear microscopy only reported observed prevalence of smear-positive disease of 39 and 45 per 100 000 [Reference Zaman33, Reference Zaman34]. The most recent national prevalence survey in the Philippines utilised the Xpert MTB/RIF® assay in addition to smear and culture, resulting in a prevalence estimate of 799 per 100 000 [28] (Tables 1–3, Fig. 2).

Fig. 2. Estimated national TB prevalence among adolescents and young people (per 100 000), from national TB prevalence surveys (country, survey start year, age group).
Table 1. Incidence of confirmed TB among young people in prospective cohort studies

Table 2. TB prevalence among young people in national prevalence surveys
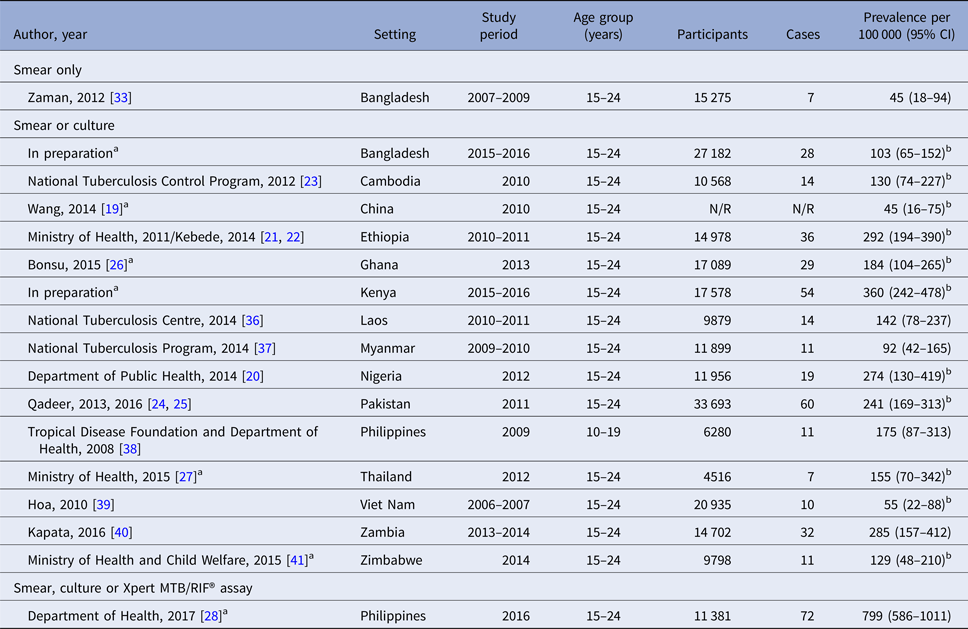
N/R, not reported.
a Personal communication of adolescent-specific data, Irwin Law (World Health Organization).
b Adjusted for clustering and for missing data using inverse probability weighting [Reference Floyd16].
Table 3. Confirmed TB prevalence among young people in sub-national prevalence surveys
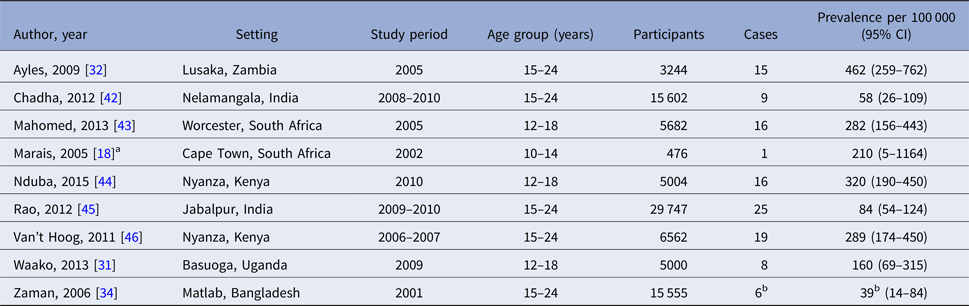
a Personal communication of adolescent-specific data, Ben Marais (University of Sydney).
b Smear-positive TB only.
Estimated TB prevalence was lowest in China at 45 per 100 000 population aged 15–24. However, as China is home to an estimated population of 237 million people aged 15–24, the estimated prevalent caseload in China remains very substantial at an estimated 106 000 prevalent cases among 15–24-year olds in 2012 (Fig. 3). Pakistan, the Philippines, Ethiopia and Nigeria all have both high prevalence and substantial estimated caseloads owing to high prevalence rates in large populations, while Laos, Cambodia and Thailand each have fewer than an estimated 15 000 prevalent cases, despite each having prevalence rate estimates in excess of 100 cases per 100 000 population.

Fig. 3. Estimated number of adolescents and young adults with prevalent pulmonary TB, based on national prevalence survey data (country, survey start year, age group).
Risk of bias
Given our restrictive exclusion criteria, the risk of bias within most included studies is considered to be low. Most studies used multiple screening strategies (most commonly symptom screening and chest X-ray), which should have moderate to high sensitivity in this age group, followed by laboratory confirmation using both smear and culture, which should provide high specificity. Four studies used symptom screening alone in some or all clusters [Reference Zaman33, Reference Zaman34, Reference Chadha42, Reference Rao45], which could reduce sensitivity and may lead to underestimates of prevalence [47]. Almost all studies used both smear and culture for bacteriological confirmation; two studies used smear without culture [Reference Zaman33, Reference Zaman34], which would have reduced sensitivity and resulted in an underestimate of the prevalence of all active pulmonary TB in their setting, Bangladesh [47].
Most studies used random sampling methods within clearly defined geographic areas, although the two cohort studies enrolled adolescents from high schools [Reference Waako31, Reference Mahomed35, Reference Mahomed43], who may not be representative of out-of-school adolescents in the community. As recorded in Table 2, not all studies presented age-specific estimates adjusted for both non-participation and clustering, although in studies where both crude and adjusted age-specific estimates were presented, the differences were usually not substantial.
Risk of bias across studies is considered to be low; publication bias should be minimal, although the delay between the undertaking and the publication of large prevalence surveys is often lengthy, and some completed studies may not yet be represented in the literature. Unsurprisingly, all the included studies were conducted in moderate or high TB transmission settings in Asia and Africa – population-based epidemiological studies of TB are not feasible in lower transmission settings, including many South American settings, as the denominators required become prohibitively large as prevalence falls.
It should be noted that sub-national studies should not be interpreted as representing the true prevalence of TB among all adolescents and young adults in the nation. India, in particular, is both very large and epidemiologically diverse with regard to TB, and estimates pertaining to the single districts in which the two Indian studies were conducted may not be nationally representative [Reference Chadha42, Reference Rao45]. Likewise, the South African studies intentionally targeted high transmission urban communities [Reference Marais18, Reference Mahomed35, Reference Mahomed43]. At the time of writing, national TB prevalence surveys were planned in India and underway in South Africa (Law, personal communication).
Discussion
This project reviewed published and unpublished data on TB incidence and prevalence among young people around the world. The identified studies were conducted in a variety of moderate- and high-transmission settings in the Asia-Pacific region and sub-Saharan Africa. The results presented demonstrate that young people in many high transmission settings currently experience a substantial burden of TB. Adolescents are often considered by both paediatric and adult clinicians to be at ‘low risk’ of TB, which may reflect the clinical experience that adolescents represent a low proportion of patients accessing either child or adult health services for TB treatment. However, results from southern Africa and the Philippines, in particular, demonstrate that although young people may be at lower risk than adults in the same country, the absolute magnitude of the risk they face in high-transmission settings can be very substantial. Further, owing to sizeable populations of people aged 10–24 years, very large numbers of young people in the Asia Pacific region are likely living with TB, even in countries such as Pakistan and China where per capita prevalence is only moderate. This is consistent with recent estimates that 1.2–3.0 million adolescents and young adults develop TB each year, a majority of whom live in the WHO African and South East Asian regions [Reference Snow14]. These findings imply a need for improved TB prevention and management in this age group, as well as consideration of their role in unfolding TB epidemics around the world.
TB prevention
While infants and young children are often exposed to TB in the household by caregivers, adolescents spend more time outside the household, where they may face an increased risk of exposure to an infection with M. tuberculosis [Reference Marais5]. Unlike young children, adolescents are at risk of exposure to disease by same-age peers [Reference Marais5, Reference Middelkoop48], a risk amplified in some settings by classroom overcrowding and poor ventilation [Reference Richardson49]. Historical data suggest that there may be a second peak in the risk of progression to active TB after infection during the adolescent years [Reference Comstock, Livesay and Woolpert7], although one smaller modern study among migrants did not observe the same phenomenon [Reference Trauer50]. Whether it is due to increased risks of infection, progression, or both, it is consistently observed that burden of disease increases with advancing age throughout adolescence [Reference Morabia8, Reference Onozaki51]. The prevalence of major risk factors for TB such as HIV, smoking, diabetes and pregnancy likewise increase with age across adolescence and young adulthood [Reference Gore12].
Notwithstanding the recognised escalation in TB risk during the adolescent years, little has been written regarding effective TB prevention interventions in the adolescent age group. There is currently no effective vaccine to prevent pulmonary TB, although several novel candidates are currently in clinical trials [Reference Zhu52]. Contact tracing and TB preventive therapy are currently routinely recommended for children under 5, but are rarely implemented even for this highly vulnerable group in moderate- and high-incidence countries [Reference Rodriguez53]. If the risk of progression to disease is indeed elevated during the adolescent years, preventive therapy for this age group might also be an efficient public health intervention; however, this is currently routinely implemented only in high-income countries in response to household contact or school-based outbreaks.
TB management
After disease develops, adolescents and their guardians may need to negotiate the space between child and adult health services [Reference Sawyer13], which can complicate the diagnostic pathway depending on whether child or adult algorithms are used. This will be doubly true in low- and moderate-incidence settings, where a variety of other respiratory diseases may be more common than TB, complicating differential diagnosis for clinicians who may see TB in adolescents only infrequently.
Once the diagnosis is achieved, treatment can be initiated; however, a wide variety of health services are challenged by engaging adolescents and young adults in on-going care [Reference Rachas54]. Supporting adherence among young people is appreciated to require specialised approaches, especially with regard to stigmatised and complex conditions, such as HIV [Reference Rachas54, Reference MacPherson55]. Comparatively little has been written regarding support for young people receiving treatment for TB: two recent studies in southern Africa have documented elevated risks of treatment discontinuation among young people with TB compared to children and adults [Reference Enane10, Reference Snow11], and an additional study from India documented complex psychosocial support needs among adolescents with multidrug-resistant TB/HIV co-infection [Reference Isaakidis9]. In settings where clinic-based direct observation of therapy remains the standard of care, TB treatment may disrupt schooling or vice versa. Infection control concerns can also result in adolescents with TB being excluded from school after treatment is initiated, sometimes unnecessarily. Although the needs of young people with TB are beginning to be appreciated, evidence on optimal models of care for TB among this group is currently absent from the literature. Lessons may be learned from other chronic conditions, where dedicated programmes targeting young people are more commonly implemented [Reference Rachas54, Reference MacPherson55].
Long-term implications
In addition to suffering a substantial burden of disease at present, our findings also imply that young people in some high-transmission settings likely have a high prevalence of M. tuberculosis infection, which may become an active disease in the distant future. Given what is known with regard to disease progression risk in this age group [Reference Marais5, Reference Trauer50], the prevalence of latent infection is likely to be 6–20 times higher than the prevalence of active disease. Some young people currently acquiring M. tuberculosis infection will ultimately contribute to TB incidence in their communities as adults over the coming decades, should they develop reactivation disease. The dramatic fall in under-five mortality in the last decade is resulting in many more children reaching adolescence [56], creating the largest generation of young people in human history [57]. The significance of this generation of young people is particularly striking in countries in the African region, where they comprise up to 30% of the population of some countries [Reference Lowenthal58]. The future course of the global TB epidemic will be determined to a significant extent by the success or failure of TB control efforts among this generation, including the prevention of both TB infection and disease.
Limitations
In spite of the widely recognised significance of age in TB epidemiology, we encountered widespread failures to report age-specific data appropriately. A large number of studies were excluded from this review because they did not report any age-stratified data. Many studies reported the age of participants simply as a mean and standard deviation, including studies targeting paediatric populations; given that the age of young TB patients often has a bimodal distribution due to the fall in incidence after early childhood and the subsequent rise during adolescence [Reference Marais5, Reference Morabia8, Reference Snow11], this is almost universally statistically inappropriate. Some studies reported age in seemingly arbitrary age groups (possibly quartiles) without explanation. Still others did not report on upper and lower bounds of age, simply referring to participants as ‘children’ or ‘adults’. This is unfortunately consistent with recent experience in reviews related to HIV in adolescents [Reference MacPherson55, Reference Lowenthal58], and hampers our understanding of the epidemiology of these two important diseases in this age group. Reporting age in standard 5-year age groups (0–4, 5–9, 10–14 and so on) in all studies of paediatric and adolescent TB and HIV, and in standard 10-year age groups for population-based studies, would address this issue.
In addition to the paucity of published age-specific data, the major limitation of the included studies and consequently of this review itself is the statistical precision of the estimates. Even national prevalence surveys with participants numbering in the tens of thousands are not powered to provide high-precision estimates of TB prevalence within narrow age bands. This is unfortunately an unavoidable issue in population-based epidemiological studies of pulmonary TB, as prevalence rarely exceeds 0.5% of a population [29].
The major strengths of this study were the broad search strategy, which should have identified a majority of relevant publications, and the inclusion of previously unpublished age-specific data from WHO records.
It was striking that we identified no eligible studies of the incidence or prevalence of confirmed TB among young people living with HIV, although the difficulties with microbiological confirmation of TB in this group present a considerable challenge to both clinicians and researchers (Rashida Ferrand, personal communication). Adolescents living with perinatally-acquired HIV infection are likely to be a high-risk group of particular importance. The generation of children born after ART became available in high HIV prevalence settings are now passing through adolescence [Reference MacPherson55, Reference Lowenthal58], with significant implications for healthcare provision. Is it estimated that there are over 2 million adolescents living with HIV around the world and that HIV-associated mortality is currently rising in this age group even as it falls in all others [59]. A key challenge among perinatally-infected young people is the risk of disengagement from HIV care if they are inadequately supported through the transition from paediatric to adult HIV services [Reference Rachas54, Reference MacPherson55, Reference Lowenthal58]. Inconsistent or interrupted access to ART for adolescents who fail to make this transition successfully is likely to increase their risk of TB. That we did not identify a single epidemiological study of confirmed TB in this group is cause for concern. Existing cohort studies planned TB prevalence surveys and other opportunities should be explored to fill this information gap, especially as higher sensitivity tests for paucibaccilary disease (e.g. the Xpert MTB/RIF assay) are now in routine use in many settings. In the meantime, for children and adolescents receiving HIV treatment and care, recommended routine TB screening activities could generate data on TB incidence and prevalence.
Conclusion
Age-specific data regarding TB epidemiology are inconsistently reported; however, several studies documented a substantial burden of TB among young people in settings in Asia and southern Africa. There is a need to improve standardised reporting of age-specific data in epidemiological studies of TB. Given the burden of disease documented in many studies included in this review, TB prevention and management in young people living in many high TB transmission settings merits increased attention.
Acknowledgements
Nathan Ford and Amitabh Suthar (World Health Organization) provided early guidance on the search strategy. Irwin Law and Ikushi Onozaki (World Health Organization (WHO)) provided valuable guidance on the design and analysis of national prevalence surveys, and Irwin Law kindly assisted in locating relevant published and unpublished data in WHO records. Ben Marais responded to a request for additional adolescent data from his study in South Africa. Rashida Ferrand provided insight into diagnostic issues among children and adolescents living with HIV. This work was funded by the NHMRC Centre for Research Excellence in Tuberculosis (TBCRE) seed funding scheme. The funder did not have any role in the design or conduct of the research. CS is a staff member of the WHO. The author alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the WHO.
Author contributions
LN and SG conceived the study. CS advised on epidemiologic measures of interest, and the inclusion and exclusion criteria. SS and SG advised on aspects of global adolescent health and the interpretation of findings. KS and LN screened titles and abstracts. KS reviewed full texts and extracted the data. All authors contributed to the manuscript.