Introduction
It is estimated that approximately 6⋅5 % of U.S. adults over age 60 suffer from dementia and 2⋅5–11 % suffer from mild cognitive impairment (MCI)(Reference DeCarli1–Reference Sosa-Ortiz, Acosta-Castillo and Prince3), resulting in significant burdens to families and the healthcare system(4). The repeated lack of success in developing effective treatments for age-related cognitive impairment necessitates examining preventative approaches. The possible role of diet in protecting against cognitive dysfunction in late life represents a potentially safe and relatively low-cost prevention strategy.
Eggs are nutritionally rich and contain many important compounds that have been found to enhance cognition, such as choline, lutein and tryptophan(Reference Naber, Hommel and Colzato5–Reference Markus, Verschoor and Firk7). Apart from the effect of individual nutrients, however, research on the effect of eating eggs, as a whole food, on cognition has been limited. Preliminary evidence suggests that dietary consumption of eggs may have a protective effect on cognition in older adults(Reference Ylilauri, Voutilainen and Lönnroos8). A major limitation of many prior studies is that analyses of egg consumption did not necessarily control for consumption of other foods, including meat and dairy, both of which are commonly eaten with eggs in the U.S. diet. The confounding effects of these other foods may have led to conflicting findings. For example, eggs have been associated with lower risk of cognitive impairment when grouped together with dairy(Reference Shin, Lee and Kim9,Reference Ashby-Mitchell, Peeters and Anstey10) , but associated with higher risk of cognitive decline when grouped together with meat, fish and poultry(Reference Tsai11). Thus, further investigation of the individual effect of eggs, apart from other food groups, is needed. In addition to dietary factors, cognition in older adults has been associated with other health-related variables, including body mass index (BMI), cardiovascular risk and depression(Reference Donovan, Wu and Rentz12–Reference Schmeidler, Mastrogiacomo and Beeri14). The present study includes rigorous controls to examine the association between egg consumption and cognition in older adults, beyond these other covariates known to have associations with cognition.
The Adventist Health Study-2 (AHS-2) is a longstanding prospective cohort study that is unique in its large variation in dietary intake of both eggs and meat, ranging from none to levels typical of an American diet. Furthermore, it is a health-conscious, aging cohort; those included here from the Biopsychosocial Religion and Health Substudy are all over age 50 and had memory assessed at two time points. This cohort is ideal to investigate the association between egg consumption and cognitive decline in the context of overall diet and consumption of other food groups. The present study tested the hypothesis that egg intake would be associated with rates of cognitive decline in memory performance independent of sociodemographic, cerebrovascular risk and other dietary factors.
Methods
Study population
The AHS-2 is a prospective cohort study comprised of 96 469 members of the Adventist Church in the USA and Canada. From February 2002 to May 2007, mostly White (65⋅3 %) and Black (26⋅9 %) adult men and women with a mean age of 59 years (range 30–110 years) were enrolled and completed a 50-page Baseline Questionnaire which included the food frequency questionnaire (FFQ) and sections on demographic, anthropometric and lifestyle factors(Reference Butler, Fraser and Beeson15). In addition to the diverse dietary patterns, the AHS-2 cohort is unique in its near absence of smoking and alcohol consumption as potential confounders (81 % of the cohort have never smoked and only 1 % are current smokers; 90 % of the cohort report no alcohol use and 5 % report consuming alcohol rarely)(Reference Orlich, Singh and Sabate16).
The present study utilised data from the AHS-2 and the AHS-2 Biopsychosocial Religion and Health Substudy (BRHS)(Reference Lee, Morton and Walters17). The BRHS biomarker study arm was designed to examine the relationship between psychosocial risks and biological indicators of allostatic load. Specifically, 536 individuals from the AHS-2 cohort were recruited in 2006–2007 to complete cognitive, physical performance, and biometric evaluation at the LLU campus and at three mobile clinics; 330 participants returned for follow-up evaluation in 2010–2011. To decrease selection bias at follow-up, home visits were completed for those too frail or ill to return to clinic for follow-up. BRHS participants who were at least 50 years old were included in the present study. Individuals reporting extreme total dietary intake of <500 or >4500 kcal/d, with missing data on the memory test or with extreme scores on the memory test at baseline were excluded.
Approvals for the AHS-2 and BRHS were obtained from the Loma Linda University Human Subjects Committee Institutional Review Board. Written informed consent was acquired from all AHS-2 and BRHS participants upon enrolment, and secondary data used in this study were de-identified.
Dietary data
Usual dietary intake during the previous year was assessed upon enrolment in the AHS-2 by a self-administered quantitative FFQ of more than 200 food items(Reference Butler, Fraser and Beeson15). The AHS-2 FFQ has been validated for foods and nutrients(Reference Jaceldo-Siegl, Fan and Sabate18,Reference Jaceldo-Siegl, Knutsen and Sabate19) in comparison to six repeated 24-h dietary recalls. Using a single item in the FFQ that assesses the frequency of eating eggs, including eggs fried, boiled, scrambled, devilled, omelette or egg salad, alone or in mixed dishes, along with eggs included in other food recipes (i.e., mayonnaise, breads, cakes, cookies), participants’ total intake of eggs was calculated and energy-adjusted using the residual method. Intake of meat, fish, dairy and fruit/vegetable food groups were also measured in g/d.
Memory performance
Participants were administered the California Verbal Learning Test – 2nd Edition, Short Form (CVLT-II), a word-list learning task that measures verbal learning and memory(Reference Delis, Kramer and Kaplan20), at both visits of the BRHS. Specifically, participants were asked to immediately recall a list of nine words over four trials. The number of words recalled on the first trial (Trial 1 Recall) represents a form of attention span reflecting one's ability to initially encode information when confronted with a large amount of information. The sum of words recalled across all four trials (Total Immediate Recall) is a general measure of one's ability to encode information. After the four learning trials, followed by a short distraction task, participants are asked to recall the list from memory (Short Delay Recall), and after an additional delay period of 10 min (after the physical performance test), participants are asked to recall the words again (Long Delay Free Recall). Finally, they are asked to try to recall additional words after being provided with category cues (Long Delay Cued Recall) and then given a yes/no recognition task (Recognition Discrimination).
Confounders
Demographic data (age, sex, race and education) were obtained at the BRHS baseline visit. Other confounders were selected from previous literature including total energy intake, dietary intake of meat, fish, dairy and fruits/vegetables(Reference Samadi, Moradi and Moradinazar21), cardiovascular disease(Reference Hooghiemstra, Leeuwis and Bertens13), BMI(Reference Schmeidler, Mastrogiacomo and Beeri14) and depressive symptoms(Reference Donovan, Wu and Rentz12). Data from the AHS-2 FFQ provided total energy (continuous) and select food group intake (meat, fish, dairy, fruits/vegetables; continuous in g/d). A cardiovascular risk (CV risk) score was calculated based on participants’ reported history of diagnosed cardiovascular disease or risk factors at baseline: a score of 2 for individuals with a reported history of CV event (i.e., stroke, transient ischaemic attack, myocardial infarction, congestive heart failure or angina pectoris); a score of 1 for individuals with CV risk factors (i.e., hypertension, hyperlipidaemia or diabetes); a score of 0 for individuals with no history of CV events or risk factors. Depression was assessed at both visits using the 11-item short form of the Center for Epidemiological Studies Depression Scale (CES-D)(Reference Kohout, Berkman and Evans22), with possible scores ranging from 0 to 22.
Statistical analyses
The present study was a secondary analysis of the AHS-2 Biopsychosocial Religion and Health Substudy (BRHS)(Reference Lee, Morton and Walters17). The data were evaluated for outliers and normality using histograms and box plots. Baseline means and percentages of demographic characteristics, dietary intake and other covariates were compared across categories of egg consumption using one-way ANOVA for normally distributed variables, Kruskal–Wallis tests for non-normal and χ 2 tests for categorical variables.
Dietary variables were energy-adjusted using the residual method. Since more than half of the subjects did not consume any meat or fish, zero intakes were partitioned out as zero and the residual method was applied on non-zero intake(Reference Jaceldo-Siegl, Fan and Sabate18). Non-zero intakes were log-transformed before energy adjustment and then transformed back to the original scaling. When meat, fish, dairy and fruit/vegetable intakes were included in regression models as covariates, transformed energy-adjusted intake log (x + 1) was used including zeros.
All analyses were conducted using R, version 3.6.1(23). A P-value of less than 0⋅05 was considered statistically significant, and all P-values were two-sided.
Results
Of the 536 participants in the BRHS cohort, 500 participants were at least 50 years old (mean 68⋅79, sd 11⋅62), and 330 returned for follow-up evaluation an average of 3⋅3 years after baseline assessment (range 2⋅7–4⋅4 years). The sample was 69 % female, and 36 % Black, 58 % White and 6 % other ethnicities. White and participants of other ethnicities were combined into a single group (‘Non-black’). Individuals reporting extreme total dietary intake of <500 or >4500 kcal/d, with missing data on the memory test or with extreme scores on the memory test at baseline were excluded. A total of thirty participants were excluded, resulting in a final sample of 470 participants (62⋅6 % female) with a mean age of 69⋅2 years (sd 11⋅3), of which 294 completed follow-up evaluation. Comparison of demographic characteristics between the final sample and excluded participants can be found in Supplementary Table S1. The thirty participants not included in the final sample were significantly younger (mean age 57⋅98, sd 12⋅83) and more likely to be Black (N 19, 63⋅3 %).
Participants were divided into three tertiles based on the amount of egg intake: Low (<23 g/week, or 0⋅5 eggs per week), Intermediate (24–63 g/week, or 0⋅5–1⋅5 egg per week) and High (≥63 g/week or 1⋅5 or more eggs per week). The Low, Intermediate and High egg consumption groups were similar in age, education, sex, race and cardiovascular risk; BMI, depression and intake of other food groups were significantly different between groups (Table 1). The Low egg intake group had lower BMI than the Intermediate and High intake groups and ate the least amount of dairy and the highest amounts of fruits and vegetables. The Intermediate egg intake group had higher depression scores than the Low and High egg intake groups, though they were still below the clinical threshold. The High egg intake group ate the highest amounts of meat, fish and dairy, and the lowest amount of fruits and vegetables. The participants who completed the memory tests at both study visits were less likely to be Black and more likely to be college graduates. There were no other significant differences between the overall sample and those who returned for follow-up (Table 2).
Table 1. Baseline characteristics of participants across categories of egg consumption

CV, cardiovascular; CES-D, Center for Epidemiological Studies Depression Scale; IQR, interquartile range.
Table 2. Baseline characteristics of participants who completed both baseline and follow-up visits
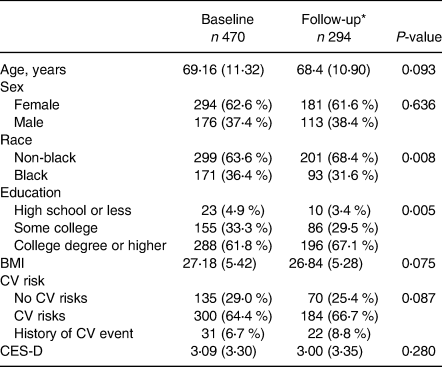
CV, cardiovascular; CES-D, Center for Epidemiological Studies Depression Scale.
* Follow-up visit was completed 3⋅3 years after baseline assessment (range = 2⋅7–4⋅4 years).
With regards to memory performance, a principal component analysis (PCA) was conducted using the baseline raw scores of the six CVLT-II tasks (Trial 1 Recall, Total Immediate Recall, Short Delay Recall, Long Delay Free Recall, Long Delay Cued Recall and Recognition Discrimination) to account for intercorrelations between variables. A correlation matrix of the six scores was used to extract PCs. The correlation matrix and results of the PCA can be found in Supplementary Tables S2 and S3, respectively. The first PC explained 70 % of the total variance among the CVLT-II variables and was used to calculate an overall summary score (CVLT-PC) as the memory outcome variable for each visit.
To examine the effect of egg intake on memory performance (CVLT-PC) and decline over time, repeated-measures analysis was conducted using a linear mixed model. The model included the three levels of egg intake (Low, Intermediate and High), age as the time variable and age by egg intake interaction terms. The age variable was centred at 50, so that its main effect tests cross-sectional differences of CVLT-PC scores among egg intake groups at this age. As a time-dependent variable, age is also paired with CVLT-PC scores at each time point in order to model cognitive decline as a function of age. The age by egg intake interactions were included to test if the rate of cognitive decline was the same across three egg intake groups. Confounders (sex, race, education, BMI, CV risk, CES-D, meat intake, fish intake, dairy intake, fruit/vegetable intake and total energy intake) were included in the model as fixed-effects terms. All these confounders were used as time-independent variables (i.e., baseline values), except for BMI and CES-D. To account for correlations among repeated measures within subjects over time, the mixed model included subjects as a random-effects term. For variables that were entered as continuous (i.e., BMI and dietary variables), the linearity assumption was tested using fractional polynomials. Other assumptions of the mixed model were assessed by visual inspection of plots of conditional residuals.
Results of the linear mixed model are presented in Table 3. As expected, age was significantly associated with CVLT-PC scores at baseline (β −0⋅05, se 0⋅007, P < 0.0001), such that increasing age was associated with lower CVLT-PC scores. After controlling for confounders, the main effects of egg intake on CVLT-PC scores were not significant, indicating no significant cross-sectional differences in memory performance between the three egg intake groups. However, there was a significant interaction effect between age and egg intake, such that the Intermediate egg intake group demonstrated a significantly slower rate of decline in CVLT-PC scores over time compared to the Low egg intake group (β 0⋅018, se 0⋅009, P 0⋅043). The High egg intake group also exhibited a slower rate of decline compared to the Low group, but the interaction term did not reach significance (β 0⋅011, se 0⋅009, P 0⋅195).
Table 3. Linear mixed model analysis of the effect of egg intake on CVLT-PC scores
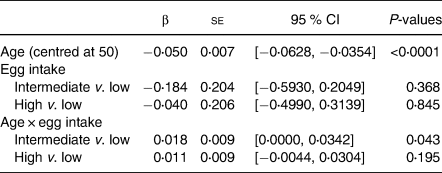
N 470.
Results are adjusted for sex, race, education, body mass index, cardiovascular risk score, Center for Epidemiological Studies Depression Scale, total energy intake, meat intake, fish intake, dairy intake, and fruit/vegetable intake.
CVLT-PC, California Verbal Learning Test-II Short Form First Principal Components Factor.
The rate of decline in the CVLT-PC score was estimated based on the fitted linear mixed model. This was calculated as a linear combination of the beta estimate associated with age and interaction terms between age and egg intake groups, with a 95 % confidence interval for each egg intake group, after adjusting for all other confounders in the model (Table 4). Similarly, the estimated CVLT-PC score at each decade was calculated for each egg intake group. The Low egg intake group was estimated to have the largest rate of decline over time among the three groups. As a result, although the estimated scores at each decade show no significant differences in memory performance at age 50 or 60, by age 70 the Low egg intake group is estimated to have the lowest CVLT-PC score of the three groups, and by age 80 it is estimated to be approximately 0⋅35 and 0⋅3 sds lower than the Intermediate and High egg intake groups, respectively.
Table 4. Estimated rates of CVLT-PC decline and estimated scores at each decade by the egg intake group
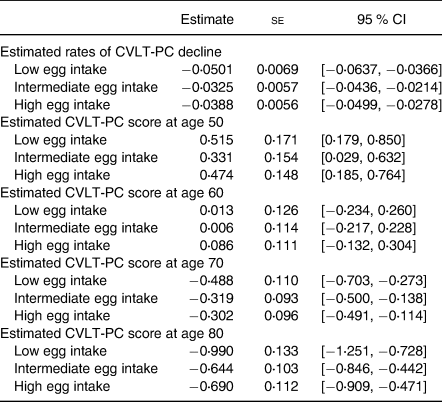
N 470.
CVLT-PC, California Verbal Learning Test-II Short Form First Principal Components Factor.
Discussion
Prior research investigating the effects of egg consumption on cognition have yielded mixed results, perhaps in part due to the variability in which egg consumption was examined in the context of other foods and dietary patterns. To our knowledge, this is the first study to examine the longitudinal associations between egg consumption and cognitive decline in older adults while controlling for other dietary factors. In our cohort of healthy, community-dwelling older adults, egg consumption had no effect on baseline memory performance. However, the amount of egg consumption did moderate the rate of age-related decline in memory performance over time. Estimated scores at each decade of life, from age 50 to 80, suggest that while egg consumption may not have a significant effect on memory function in younger decades, i.e., age 50 and 60, it may have a more long-term association with rates of memory decline in later decades, beginning at age 70.
Previous studies have shown that older adults reporting higher egg intake demonstrated better cognition across multiple areas(Reference Ylilauri, Voutilainen and Lönnroos8,Reference Aparicio Vizuete, Robles and Rodríguez-Rodríguez24,Reference Zhao, Yuan and Feng25) . Results from the Kuopio Ischemic Heart Disease Risk Factor Study, one of only a few longitudinal studies that followed its participants over a long period of time, found that higher egg intake was associated with better performance on measures of processing speed and executive function, and was also marginally associated with a lower risk of incident dementia. Other studies have reported inconsistent findings due to varying measures of dietary patterns, making it difficult to determine whether the effects are specifically related to eggs or to an overarching dietary pattern. In a cross-sectional study examining 239 older adults over age 65, individuals following a diet that was characterised by high intake of egg, along with seafood, vegetables, dairy and other foods, had a lower risk of MCI, compared to the other two diet patterns characterised by either high meat intake or high bread, ham and alcohol consumption, respectively(Reference Shin, Lee and Kim9). Similarly, in a cohort of 577 older adults from the Australian Diabetes, Obesity and Lifestyle Study who were followed over 12–13 years, a dietary pattern characterised by egg, dairy and cereal intake was associated with reduced risk of cognitive impairment(Reference Ashby-Mitchell, Peeters and Anstey10). However, other studies in which eggs were examined collectively with meat, fish and poultry intake found either no effect or an increased risk of cognitive decline(Reference Tsai11,Reference Pilleron, Desport and Jésus26) . Our study clarifies previous findings by examining the independent effect of eggs alone, while controlling for intake of these other food groups which have been frequently grouped together with eggs in previous studies.
Notably, due to the relatively large proportion of individuals in our cohort who follow a vegan or vegetarian diet compared to the general population, the actual amount of eggs consumed in our participants is relatively small. On average, the Low intake group consumed what is equivalent to less than half an egg per week, the Intermediate intake group about one egg per week, while the High intake group consumed three eggs per week. Although the memory effects of the Intermediate and High egg consumption in our cohort were similar, the effect of the High consumption group was not statistically significant; thus, there is not a clear dose-response effect of egg consumption on memory. Given that the protective effect of eggs was stronger in the Intermediate intake group than the High intake group, it is unclear if even higher amounts of egg consumption will also have the effect of mitigating age-related declines in memory. Despite the relatively small amount of eggs consumed in our cohort, we found that consuming as little as one egg a week was associated with a slower rate of memory decline in late life compared to individuals who consumed almost no eggs.
The objective of the present study was to examine the effect of the whole food rather than the effect of any specific nutrient. Nevertheless, the cognitive benefits of egg consumption may be related to eggs’ high content of choline, lutein and tryptophan, each of which have been linked to improved cognitive function(Reference Naber, Hommel and Colzato5–Reference Markus, Verschoor and Firk7). Clinical trials have shown that choline supplementation in healthy adults resulted in improved performance on tasks of processing speed, working memory, memory and visuomotor skill(Reference Naber, Hommel and Colzato5,Reference Knott, de la Salle and Choueiry27) . Choline has also been found to improve both cognitive and functional outcomes in patients up to 12 months after stroke(Reference Alvarez-Sabín, Ortega and Jacas28). Both clinical trials and observational studies have similarly found a positive association between levels of lutein and cognitive performance in attention, memory and executive function(Reference Feeney, O'Leary and Moran29,Reference Hammond, Miller and Bello30) . Tryptophan treatment has been shown to improve performance on tasks of perceptual motor, speed and attention(Reference Mohajeri, Wittwer and Vargas6,Reference Markus, Verschoor and Firk7) , and low blood levels of tryptophan are associated with greater cognitive impairments in non-demented, community-dwelling older adults(Reference Solvang, Nordrehaug and Tella31,Reference Ramos-Chávez, Roldán-Roldán and García-Juárez32) . Tryptophan is also a precursor to serotonin, a neurotransmitter implicated in depression, which has been independently associated with an increased risk of cognitive impairment and dementia in older adults(Reference Lee, Lu and Hua33,Reference Teng, Lu and Cummings34) . Accordingly, tryptophan-enriched egg white protein formulations have been found to improve mood and inhibit the cortisol response to acute stress(Reference Firk and Markus35). Thus, eggs may serve as an important source of nutrients that have both direct and indirect effects (through depression) on brain function.
Strengths of this study are a relatively large sample of healthy older participants who exhibit a wide range of dietary patterns, and generally avoid smoking and alcohol consumption, neurotoxins known to impair memory. Dietary intake was assessed with a validated and detailed questionnaire. In addition to demographic variables, many important health covariates were also assessed at the same time as memory that allowed for more rigorous adjustments. Moreover, the confounding effects of other foods were also controlled for. Limitations include the inability to draw causal conclusions based on a secondary analysis of an observational study. Also, the range of egg intake in this cohort is lower than the general population and may therefore underestimate the association between eggs and memory decline.
In conclusion, the present study found that even limited egg intake (about one egg a week) was associated with slower rates of memory decline over a period of 3–4 years compared to consuming little to no eggs. Our findings provide support for a protective effect of egg consumption on memory function, particularly after age 70. The present study may help explain discrepancies in previous research that did not control for other dietary intakes and risk factors. Future research should focus on investigating the effect of higher egg intake on memory decline in late life.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/jns.2021.76.
Acknowledgement
The Biopsychosocial Religion and Health Study and parent Adventist Health Study-2 study were funded by the National Institute on Aging (1R01AG026348) and the National Cancer Institute (1U01CA152939), respectively. The present analysis was funded by the American Egg Board (AEB). The funding organisations had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
The authors’ contributions were as follows – J.S. and K.R.M. contributed to the research design; K.R.M. and M.O. conducted research; K.O. performed statistical analyses; G.J.L., J.S., K.O., K.R.M. and M.O. interpreted the data; G.J.L. drafted the manuscript and had primary responsibility for final content. All authors provided critical revisions to the manuscript, and read and approved the final manuscript.
Approvals for the AHS-2 and BRHS were obtained from the Loma Linda University Human Subjects Committee Institutional Review Board. All study procedures were performed in accordance with the ethical standards of the Institutional Review Board and the Declaration of Helsinki.
The authors have no conflicts of interest to disclose.








