Introduction
Insect herbivory on fossilized leaves (hereafter “fossil herbivory”) has been noted incidentally for more than 100 years (Potonié Reference Potonié1893). However, the systematic collection of herbivory data only came with the advent of the Damage Type system (Wilf and Labandeira Reference Wilf and Labandeira1999), in which each type of insect damage—for example, circular holes below 1 mm in diameter, circular holes between 1 and 5 mm in diameter—is assigned a unique number and is classified into a broader functional feeding group (Labandeira et al. Reference Labandeira, Wilf, Johnson and Marsh2007).
Traditionally, quantitative analyses of fossil herbivory have focused on two topics: the richness of damage types in a fossil assemblage or for a particular host plant (Wilf and Labandeira Reference Wilf and Labandeira1999) and the intensity of insect damage as measured by the percentage of leaf area removed by herbivores (Beck and Labandeira Reference Beck and Labandeira1998). Another layer of biological and analytical complexity can be added by linking particular host plants to particular damage types. On the one hand, quantitative methods in paleontology and ecology have progressed tremendously during the past two decades, making it possible to conduct complex analyses of fossil herbivory data with a single line of code after loading a software package. On the other hand, such analyses require more complete datasets than are typically available in studies of fossil herbivory. Many newly possible analyses also rely upon more assumptions about biological processes and data structure or estimate more parameters than do traditional analyses. In such cases, the underlying assumptions and their effects can become more difficult to identify and address.
Research Topics That Link Host Plants to Damage Types
Three interrelated research topics link host plants to damage types: host specificity, component communities, and compound communities. Host specificity differentiates among generalist and specialist feeding strategies. A component community is the entire suite of heterotrophs that rely, directly or indirectly, on a plant taxon: its herbivores and their predators, parasitoids, and parasites (Root Reference Root1973). A suite of coexisting component communities, that is, those of the different plant species within the same forest, is called a “compound community” (Reice Reference Reice1974; Whittaker and Levin Reference Whittaker and Levin1977; Basset Reference Basset1992; Novotny et al. Reference Novotny, Basset, Miller, Drozd and Cizek2002). All of these topics present challenges when translated to the fossil record.
The host specificity of each fossil insect damage type is typically measured on a scale of 1 to 3 (Labandeira et al. Reference Labandeira, Wilf, Johnson and Marsh2007). Generalized damage types, occurring on a range of distantly related plant hosts, have a score of 1. Damage types of intermediate specificity have a score of 2. Specialized damage types, restricted to very closely related plant hosts, have a score of 3. These scores are assigned to damage types that occur on three or more specimens in a fossil assemblage. The assignment of these scores at various fossil assemblages is difficult to replicate, because the boundaries between the scores are not defined quantitatively—these symbols are merely qualitative and contain no quantitative information—but the many datasets that have become available since 1999 can be used for sensitivity analyses to evaluate the validity and reliability of this system.
For component communities, identification of the secondary consumers associated with the herbivores on a host plant is challenging with fossils (Greenwood Reference Greenwood and Donovan1991; Martínez-Delclòs and Martinell Reference Martínez-Delclòs and Martinell1993; Smith and Moe-Hoffman Reference Smith and Moe-Hoffman2007). Many of the iconic insect Lagerstätten also contain abundant plant fossils (from oldest to youngest: Carpenter Reference Carpenter, Shabica and Hay1997; Wittry Reference Wittry2006; Novokshonov Reference Novokshonov1997; Ponomareva et al. Reference Ponomareva, Ponomarenko and Naugolnykh1998; Cairncross and Anderson Reference Cairncross and Anderson1995; Anderson Reference Anderson1999; Dobruskina Reference Dobruskina1995; Shcherbakov Reference Shcherbakov2008; Huang et al. Reference Huang2016; Ren et al. Reference Ren, Shih, Gao, Wang and Yao2019; Huang Reference Huang2016; Xiao et al. Reference Xiao, Labandeira and Ren2022; Ribeiro et al. Reference Ribeiro, Ribeiro, Varejão, Battirola, Pessoa, Simões, Warren, Riccomini and Poyato-Ariza2021; Wappler et al. Reference Wappler, Currano, Wilf, Rust and Labandeira2009; MacGinitie Reference MacGinitie1969; Wilson Reference Wilson1978; Grande Reference Grande1984; Dayvault et al. Reference Dayvault, Codington, Kohls, Hawes, Ott and Behnke1995; Wappler et al. Reference Wappler, Labandeira, Rust, Frankenhäuser and Wilde2012; Dunne et al. Reference Dunne, Labandeira and Williams2014; Douglas and Stockey Reference Douglas and Stockey1996; Labandeira Reference Labandeira2002; Constenius et al. Reference Constenius, Dawson, Pierce, Walter and Wilson1989; Greenwalt and Labandeira Reference Greenwalt and Labandeira2013; Wilde and Frankenhäuser Reference Wilde and Frankenhäuser1998; Lutz et al. Reference Lutz, Kaulfuss, Wappler, Loehnertz, Wilde, Mertz, Mingram, Franzen, Frankenhaeuser and Koziol2010; Wappler et al. Reference Wappler, Labandeira, Rust, Frankenhäuser and Wilde2012; Wilson Reference Wilson1978; Meyer Reference Meyer2003; Allen et al. Reference Allen, Lowe, Peppe and Meyer2020). When the relevant plants and insects do co-occur, it is nearly impossible to link a particular insect taxon (within a given feeding guild) to a particular damage type (within a given functional feeding group). Nonetheless, component communities in the fossil record have been widely discussed using damage types as proxies for herbivore taxa (Labandeira Reference Labandeira1998, Reference Labandeira2002; D'Rozario et al. Reference D'Rozario, Labandeira, Guo, Yao and Li2011; Slater et al. Reference Slater, McLoughlin and Hilton2012, Reference Slater, McLoughlin and Hilton2015; Labandeira and Currano Reference Labandeira and Currano2013; Labandeira et al. Reference Labandeira, Tremblay, Bartowski and VanAller Hernick2013, Reference Labandeira, Kustatscher and Wappler2016, Reference Labandeira, Anderson, Anderson and Tanner2018; Ding et al. Reference Ding, Labandeira and Ren2014, Reference Ding, Labandeira, Meng and Ren2015; Schachat et al. Reference Schachat, Labandeira, Gordon, Chaney, Levi, Halthore and Alvarez2014, Reference Schachat, Labandeira and Chaney2015; Feng et al. Reference Feng, Wang, Rößler, Ślipiński and Labandeira2017; Kustatscher et al. Reference Kustatscher, van Konijnenburg-van Cittert, Looy, Labandeira, Wappler, Butzmann, Fischer, Krings, Kerp and Visscher2018; Xu et al. Reference Xu, Jin and Labandeira2018; Correia et al. Reference Correia, Bashforth, Šimůnek, Cleal, Sá and Labandeira2020; Liu et al. Reference Liu, Wei, Chen, Guo, Zhou, Gou, Yang, Labandeira and Feng2020). However, here too, there is reason for caution: even the fossil floras that have been most thoroughly sampled for insect herbivory contain various damage types that occur on only one specimen (Wilf et al. Reference Wilf, Labandeira, Johnson and Cuneo2005, Reference Wilf, Labandeira, Johnson and Ellis2006; Prevec et al. Reference Prevec, Labandeira, Neveling, Gastaldo, Looy and Bamford2009; Wappler Reference Wappler2010; Knor et al. Reference Knor, Prokop, Kvaček, Janovský and Wappler2012; Wappler et al. Reference Wappler, Labandeira, Rust, Frankenhäuser and Wilde2012; Donovan et al. Reference Donovan, Wilf, Labandeira, Johnson and Peppe2014; Adroit et al. Reference Adroit, Girard, Kunzmann, Terral and Wappler2018; Labandeira et al. Reference Labandeira, Anderson, Anderson and Tanner2018; Xu et al. Reference Xu, Jin and Labandeira2018; Deng et al. Reference Deng, Su, Wappler, Liu, Li, Huang, Tang, Low, Wang, Xu, Xu, Liu and Zhou2020), indicating that many damage types remain unobserved due to incomplete preservation and sampling. Because we cannot find every damage type from a fossil assemblage, and because we cannot link damage types to the insect taxa in a one-to-one manner, the term “component community” as developed in the context of modern ecology may be somewhat inapplicable. These issues then scale up to consideration of compound communities.
Whereas convincingly complete sampling is hardly inevitable in the modern, it is at least possible. This can be seen in a recent comparison of fossil and modern herbivory (Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022). For one of the modern datasets considered, 1500 m2 of leaf area was sampled for each plant species (Novotny et al. Reference Novotny, Miller, Hrcek, Baje, Basset, Lewis, Stewart and Weiblen2012). For another modern dataset, between 1500 and 10,500 m2 of leaf area was sampled for each plant species (Novotny et al. Reference Novotny, Miller, Basset, Cizek, Darrow, Kaupa, Kua and Weiblen2005). In contrast, between 0.000497 and 1.62 m2 of leaf surface area was sampled for each fossil plant taxon included in the study. Relative to the four paleontological studies, the amount of leaf area examined in the two highlighted modern studies is at least 925 times greater, and can be more than 20 million times greater.
Despite these issues, the general concepts drawn from modern ecology that underlie discussions of component communities in the fossil record are nevertheless valid. Ancient plants surely had specialist and generalist herbivores that formed component communities along with their secondary consumers on each plant host species. Thus, these concepts are worthy of consideration, although we must be wary of the fidelity with which those communities might be documented in the fossil record. In particular, bipartite network analysis has recently been applied to fossil herbivory datasets to address questions about host specificity and component communities (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021; Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022). Bipartite networks may be used to connect taxa at two trophic levels, such as plants and their herbivores or herbivores and their parasitoids. Alternatively, beta diversity (Baselga Reference Baselga2010, Reference Baselga2017; Baselga and Orme Reference Baselga and Orme2012) and rarefaction of interactions (Dyer et al. Reference Dyer, Walla, Greeney, Stireman III and Hazen2010) can be used to examine herbivore specialization and component communities based on the leaf damage record. Calculating the beta diversity of damage types on different host plants is a straightforward way to compare component communities. Rarefying interactions is a straightforward way to quantify the diversity of associations within a compound community. Here, these alternatives are evaluated through sensitivity analyses to determine how much sampling is required for stable results, with the aim of ascertaining whether and how quantitative methods can be used to evaluate host specificity, component communities, and compound communities in studies of fossil herbivory. Bipartite network analysis requires special consideration because of the assumptions it requires of the fossil record and because of the risks associated with the large number of metrics that are generated.
Theoretical Issues with Bipartite Network Analysis
Treating Damage Types as Analogues of Herbivore Taxa
Methods that link particular host plants to particular damage types often treat damage types as analogues for herbivorous insect taxa. For example, the two recent studies that performed bipartite network analysis on fossil herbivory data (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021; Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022) used a software package (bipartite; Dormann et al. Reference Dormann, Gruber and Fründ2008) intended for modern ecological networks that requires direct substitution of damage types for herbivore taxa—constituting an explicit, specific assumption that has not been substantiated and likely never can be. Only one study has used neontological data to evaluate the correlation between damage types and herbivores (Carvalho et al. Reference Carvalho, Wilf, Barrios, Windsor, Currano, Labandeira and Jaramillo2014). In two tropical forests, the diversities of damage types and insect herbivores were found to be correlated, reaffirming the value of the traditional paleontological metric of damage type diversity. However, no claim was made as to whether the apparent specialization of a damage type reliably indicates whether the damage type was produced by a specialist herbivore.
Simple arithmetic supports the idea that specialized herbivores are responsible for many occurrences of “generalized” damage types: with hundreds of thousands of herbivorous insect species and only a few hundred damage types, no one-to-one correspondence between insect species and damage types is possible. For example, DT012, the most common type at both forests studied by Carvalho et al. (Reference Carvalho, Wilf, Barrios, Windsor, Currano, Labandeira and Jaramillo2014), was found on all 12 host plant species examined and was caused by 50 insect species (46 of them specialists) in one locality and 37 insect species (23 of them specialists) in the other. All that complexity is collapsed into a single generalist when fossil damage types are treated as substitutes for actual herbivores.
“Trophic species” are occasionally used as substitutes for consumer taxa in studies of ecological networks. Dunne and colleagues explain that trophic species are generated “by aggregating taxa with the exact same set of predators and prey” (Dunne et al. Reference Dunne, Labandeira and Williams2014). In other words, trophic species are “functional groups of all organisms in a web that appear to share the same set of consumer and resource species” (Memmott et al. Reference Memmott, Martinez and Cohen2000). In the context of insect herbivory, a trophic species would be a group of herbivore taxa, however distantly related, that feed on the same host plant taxon in a similar manner—and share the same predators and parasitoids. As can be seen in the preceding discussion, the aggregation into a single unit (a damage type) of specialist herbivore taxa that cause morphologically similar damage on disparate host plant taxa violates the trophic species concept. Moreover, various workers disagree with the use of trophic species (Pringle and Hutchinson Reference Pringle and Hutchinson2020).
Sampling Incompleteness
All sampling of the fossil record is incomplete, but methods that link particular host plants to particular damage types are far more biased by incomplete sampling than are the methods that address the diversity and intensity of insect herbivory. For a tally of the number of insect damage types on two host plant taxa, as an example, the more completely sampled host could be iteratively subsampled down to the amount of surface area or sample coverage available for the less completely sampled host plant (Fig. 1A). Although the subsampling procedure might cause a failure to detect a significant difference that would become apparent with additional sampling, any significant differences observed among the subsampled damage type diversities are likely, although not guaranteed, to reflect true differences. Thus, estimating damage type diversity by subsampling two incompletely sampled host plants is a common and uncontroversial endeavor. We do not know which specific damage types evaded detection, but we do not need to know this in order to estimate the damage type diversities of these two host plants when subsampled to the same surface area or sample coverage.
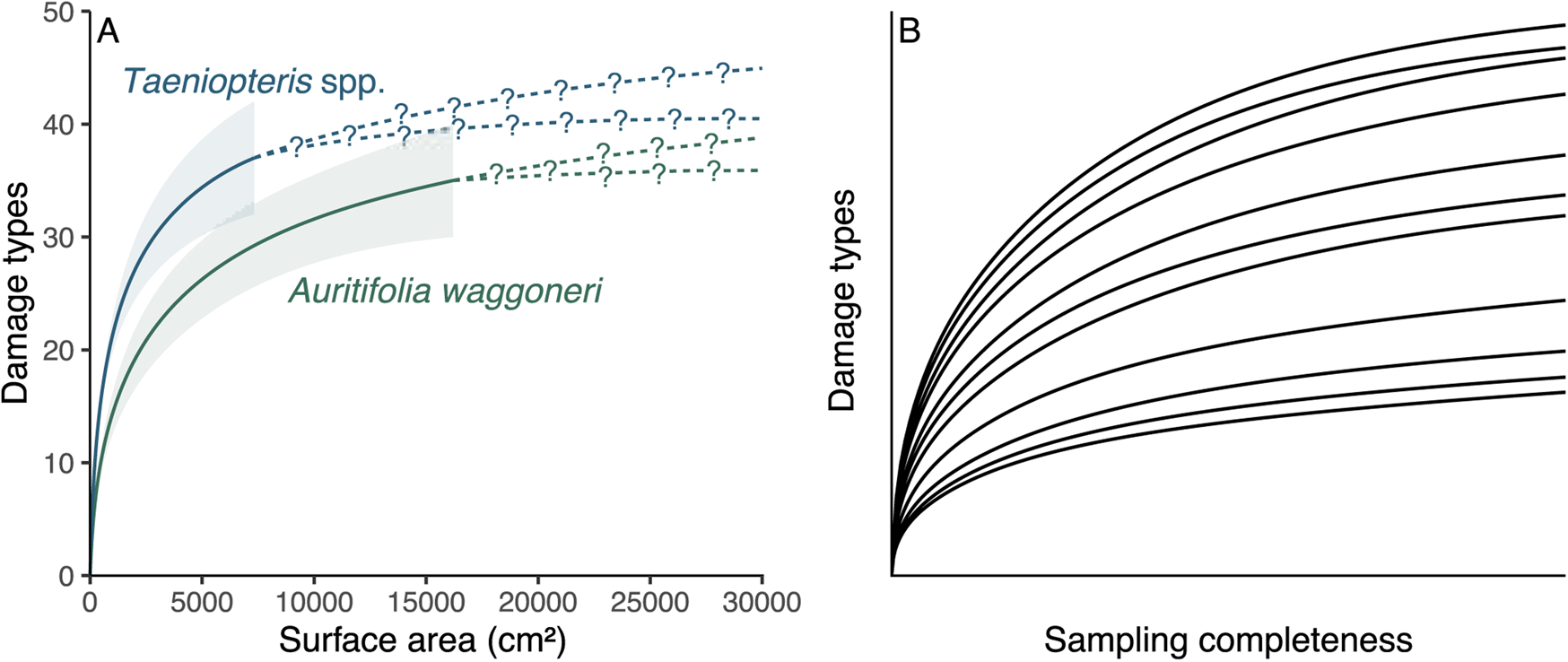
Figure 1. A comparison of the sampling completeness that can be expected for studies of fossil herbivory (A) with the sampling completeness needed for methods that link host plants to damage types to be unbiased by sampling completeness (B). A, Rarefaction of damage types on the two dominant host plants at the Colwell Creek Pond assemblage. The solid lines and corresponding 84% confidence intervals represent interpolated damage type diversity, and the dashed lines with question marks represent extrapolated diversity. B, An illustration of the sampling completeness that is needed for bipartite network analysis not to be biased by sampling: the rarefaction curve for each host plant should have sample coverage sensu Chao and Jost (Reference Chao and Jost2012) above 0.99. All rarefaction curves shown in this panel have coverage between 0.995 and 0.997.
When it comes to estimating host specificity or comparing component communities, however, the unknowable identities of unobserved damage types are of paramount importance. According to the criteria that have traditionally been used to assign host-specificity scores (Wilf and Labandeira Reference Wilf and Labandeira1999), a damage type must occur on only three specimens in order to receive such a score. The data are taken at face value, and the appearance of a damage type on three leaves is deemed adequate to designate a damage type as specialized, regardless of the possibility that a fourth or fifth observation might occur on a different host and thus change the host-specificity score. The procedures used to compare component communities are incapable of distinguishing a true absence of a damage type on a host plant from the failure to detect a damage type that was present on the host. Differentiating true absences from failures to detect is known to pose tremendous difficulties in both neontological (Blasco-Moreno et al. Reference Blasco-Moreno, Pérez-Casany, Puig, Morante and Castells2019) and paleontological (Smith et al. Reference Smith, Handley and Dietl2022) studies.
Attempts to compare host specificity and component communities across different assemblages complicate matters even further. As an example drawn from Permian assemblages of Texas for which damage type data are available for each specimen, the amount of broadleaf area examined from Colwell Creek Pond (Schachat et al. Reference Schachat, Labandeira, Gordon, Chaney, Levi, Halthore and Alvarez2014) is approximately 4 times that of Williamson Drive (Xu et al. Reference Xu, Jin and Labandeira2018) and more than 15 times that of Mitchell Creek Flats (Schachat et al. Reference Schachat, Labandeira and Chaney2015) or South Ash Pasture (Maccracken and Labandeira Reference Maccracken and Labandeira2020). There is just no good way to compare host specificity and component communities across these assemblages, because subsampling Williamson Drive and Colwell Creek Pond down to the amount of surface area examined at Mitchell Creek Flats and South Ash Pasture will fundamentally change the relationships among host plants and their damage types. At Colwell Creek Pond, DT014 has been observed on 2 Auritifolia waggoneri Chaney, Mamay, DiMichele & Kerp, Reference Chaney, Mamay, DiMichele and Kerp2009 specimens and on 20 Taeniopteris spp. Brongniart, Reference Brogniart1828 specimens. DT247 has been observed on 15 A. waggoneri specimens and 2 Taeniopteris spp. specimens. If the data from Colwell Creek Pond are subsampled to one-fifteenth of the original amount of surface area, the specificity coding of the damage types that are still observed at this lower level of sampling will fundamentally change: various damage types will appear more specialized than they are, and in many dimensions, the component communities of the two dominant host plants will appear more distinct than they are.
In the words of Blüthgen et al. (Reference Blüthgen, Fründ, Vázquez and Menzel2008: p. 3387), “rarely observed species are inevitably regarded as ‘specialists,’ irrespective of their actual associations, leading to biased estimates of specialization.” For rarefied damage type diversity and for the intensity of herbivory, the results generated at lower levels of sampling completeness are simply a less precise, underpowered version of the results generated at higher levels of sampling completeness (Schachat et al. Reference Schachat, Labandeira and Maccracken2018). For component communities, however, the results generated with less sampling are fundamentally changed. Indeed, misleading results when sampling is not exhaustive are exactly what biologists found when they subsampled some of the canonical datasets that have been used to construct bipartite networks (Morris et al. Reference Morris, Gripenberg, Lewis and Roslin2014: fig. 3) as part of the large body of work that has emerged to evaluate how incomplete sampling biases bipartite network metrics (Goldwasser and Roughgarden Reference Goldwasser and Roughgarden1997; Vázquez and Aizen Reference Vázquez and Aizen2003; Blüthgen et al. Reference Blüthgen, Menzel and Blüthgen2006, Reference Blüthgen, Fründ, Vázquez and Menzel2008; Dormann et al. Reference Dormann, Fründ, Blüthgen and Gruber2009; Dorado et al. Reference Dorado, Vázquez, Stevani and Chacoff2011; Gibson et al. Reference Gibson, Knott, Eberlein and Memmott2011; Costa et al. Reference Costa, da Silva, Ramos and Heleno2016; Fründ et al. Reference Fründ, McCann and Williams2016; Jordano Reference Jordano2016; Kuppler et al. Reference Kuppler, Grasegger, Peters, Popp, Schlager and Junker2017; Maia et al. Reference Maia, Nascimento and Faria2018; Henriksen et al. Reference Henriksen, Chapple, Chown and McGeoch2019).
A related pitfall of bipartite network analysis that looms large in the neontological literature may well be insurmountable for studies of fossil herbivory: sampling evenness. Before the construction of bipartite networks, the sampling of fossil leaves for insect damage types should be not only complete at the level of the assemblage but should be similarly complete across all host plants within the assemblage—that is, sampling of all host plants under consideration should be even (Gibson et al. Reference Gibson, Knott, Eberlein and Memmott2011; Doré et al. Reference Doré, Fontaine and Thébault2021). In studies of modern communities, sampling evenness can be achieved in various ways, for example, equal amounts of time being dedicated to hand-collecting of insects and equal numbers of beating samples collected for each of 10 tree species (Basset et al. Reference Basset, Samuelson and Miller1996) and equal amounts of surface area sampled for each plant species (Novotny et al. Reference Novotny, Miller, Hrcek, Baje, Basset, Lewis, Stewart and Weiblen2012). However, exhaustive sampling of all host plant taxa under consideration is a near impossibility for studies of fossil herbivory (Fig. 2). Most species in a given community are rare (Diserud and Engen Reference Diserud and Engen2000), and many if not most studies of fossil herbivory have examined fewer than 1000 leaves due to a combination of small numbers of specimens preserved in the fossil record and limited time that investigators can invest in each study. Therefore, in studies of fossil herbivory, most plant hosts are represented by a maximum of a few hundred leaves.
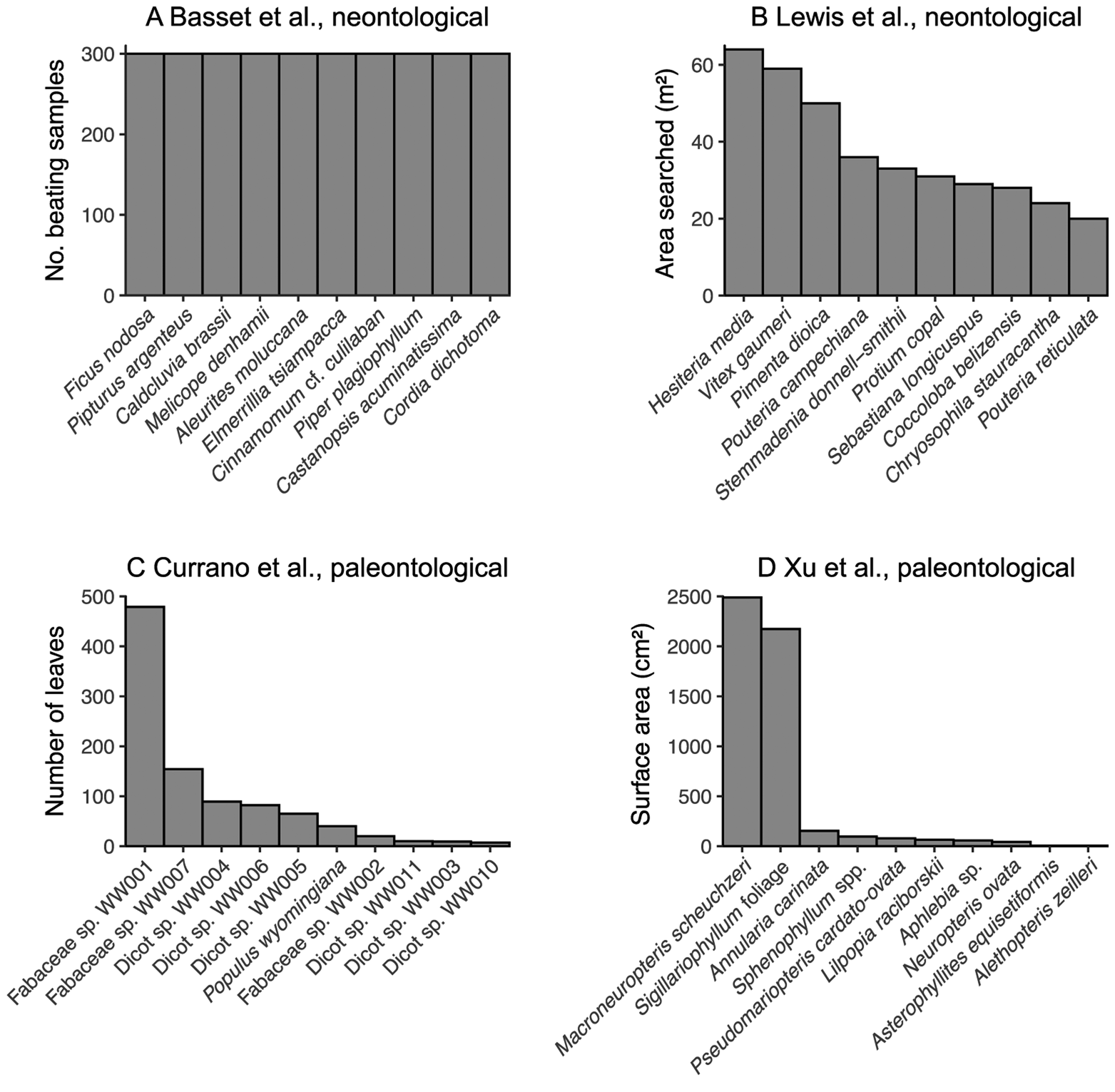
Figure 2. The sampling evenness for host plants in neontological (A, B) and paleontological (C, D) datasets that can be used to link host plants to herbivores or damage types. A, Basset et al. (Reference Basset, Samuelson and Miller1996); this maximally even sampling is representative of various other neontological studies of plant–insect networks (Novotny et al. Reference Novotny, Basset, Miller, Drozd and Cizek2002, Reference Novotny, Miller, Leps, Basset, Bito, Janda, Hulcr, Damas and Weiblen2004, Reference Novotny, Miller, Hrcek, Baje, Basset, Lewis, Stewart and Weiblen2012; Lundgren and Olesen Reference Lundgren and Olesen2005; Olesen et al. Reference Olesen, Bascompte, Elberling and Jordano2008; Pinheiro et al. Reference Pinheiro, de Abrão, Harter-Marques and Miotto2008; Gibson et al. Reference Gibson, Knott, Eberlein and Memmott2011; Grass et al. Reference Grass, Berens, Peter and Farwig2013; Trøjelsgaard et al. Reference Trøjelsgaard, Jordano, Carstensen and Olesen2015; Oleques et al. Reference Oleques, Vizentin-Bugoni and Overbeck2019; Zemenick et al. Reference Zemenick, Vanette and Rosenheim2021). B, Lewis et al. (Reference Lewis, Memmott, Lasalle, Lyal, Whitefoord and Godfray2002). C, Currano et al. (Reference Currano, Wilf, Wing, Labandeira, Lovelock and Royer2008). D, Xu et al. (Reference Xu, Jin and Labandeira2018).
Combining the concepts of sampling completeness and evenness, Morris et al. (Reference Morris, Gripenberg, Lewis and Roslin2014) recommended constructing bipartite networks for datasets in which all rarefaction curves—in this case, damage type diversity curves for all host plants—asymptote (Fig. 1B). Various neontological food web studies have affirmed and followed this recommendation (e.g., Smith-Ramírez et al. Reference Smith-Ramírez, Martinez, Nuñez, González and Armesto2005; Burkle and Irwin Reference Burkle and Irwin2009; Mokam et al. Reference Mokam, Djiéto-Lordon and Bilong2014; Kemp and Ellis Reference Kemp and Ellis2017; Peguero et al. Reference Peguero, Bonal, Sol, Muñoz, Sork and Espelta2017; Arceo-Gómez et al. Reference Arceo-Gómez, Alonso, Ashman and Parra-Tabla2018; Bennett et al. Reference Bennett, Thompson, Goia, Feldmann, Ştefan, Bogdan, Rakosy, Beloiu, Biro, Bluemel, Filip, Madaj, Martin, Passonneau, Kalisch, Scherer and Knight2018; Maia et al. Reference Maia, Nascimento and Faria2018). However, this is not nearly as easily achieved with paleontological data as with neontological data. Whereas one might question whether it is possible for a rarefaction curve to truly asymptote, the concept of “sample coverage” sensu Chao and Jost (Reference Chao and Jost2012) provides a measure of the slope of a rarefaction curve: when the curve has reached an asymptote, its slope equals 0 and coverage equals 1. For our purposes, sample coverage above 0.99 will be considered complete. If a paleontological dataset with 10 or more host plants that have coverage above 0.99 eventually becomes available, it can be used to evaluate whether slightly lower amounts of coverage continue to yield reliable results. The Appendix lists examples of host plants that have been censused for fossil herbivory for which sample coverage of damage types is above 0.99.
The requirement that all rarefaction curves reach an asymptote is unrealistic for essentially the entirety of the fossil record of insect herbivory as it is currently sampled. At the Willershausen assemblage (Adroit et al. Reference Adroit, Girard, Kunzmann, Terral and Wappler2018), for which more than 7000 angiosperm leaves were examined, coverage for the 10 most abundant host plants ranges from 0.90 to 0.99. However, at Castle Rock (Wilf et al. Reference Wilf, Labandeira, Johnson and Ellis2006), another of the few assemblages with more than 2000 angiosperm leaves examined for which damage type data are available for each specimen, coverage of the top 10 host plants is much lower, with some taxa preserving no damage at all and the highest coverage only reaching 0.72. At the Bílina–DSH assemblage (Knor et al. Reference Knor, Prokop, Kvaček, Janovský and Wappler2012), also with more than 2000 angiosperm leaves examined, coverage of the top 10 host plants ranges from 0.59 to 0.90. Therefore, low sample coverage of damage types for individual host plants is clearly not due to lack of investigator effort; this is a characteristic of some of the best-sampled assemblages. Rather, low sample coverage of damage types for individual host plants is a near-inevitability given the vastly uneven frequencies of both host plants and damage types in fossil assemblages. Even the less common host plants must be represented by enough specimens for their individual damage diversity rarefaction curves to asymptote.
HARKing
A “reproducibility crisis” in science (O'Boyle et al. Reference O'Boyle, Banks and Gonzalez-Mulé2017; Fraser et al. Reference Fraser, Parker, Nakagawa, Barnett and Fidler2018; Hutson Reference Hutson2018; Parker et al. Reference Parker, Fraser and Nakagawa2019; Bissonette Reference Bissonette2021; Nelson et al. Reference Nelson, Ichikawa, Chung and Malik2021; O'Dea et al. Reference O'Dea, Parker, Chee, Culina, Drobniak, Duncan, Fidler, Gould, Ihle, Kelly, Lagisz, Roche, Sánchez-Tójar, Wilkinson, Wintle and Nakagawa2021) has reinforced the need for caution surrounding practices such as multiple comparisons and hypothesizing after the results are known (HARKing). When the popular R package bipartite is used with its default settings to study plant–herbivore interactions, the networklevel function calculates 47 bipartite network metrics and the grouplevel function calculates 30 metrics: 15 for each host plant taxon and 15 for each herbivore taxon (Dormann et al. Reference Dormann, Gruber and Fründ2008)—77 metrics despite few studies addressing 77 distinct questions. Such a multitude of metrics raises the risk of spurious correlations whereby a small minority of metrics support preconceived notions by chance.
For bipartite network studies, calculating a single bipartite network metric per study has been recommended to avoid “metric hacking,” that is, the “nonmutually exclusive use of multiple network metrics that are correlated by variables held in common (e.g., number of host plant taxa or sampling completeness) and the inflation of type I error rates as a result of indiscriminate selection of network metrics, comparisons or hypotheses after analyses have been conducted” (Webber et al. Reference Webber, Schneider and Vander Wal2020: p. 110). This warning echoes concerns raised more than a decade earlier: “Network analyses of mutualistic or antagonistic interactions between species are very popular, but their biological interpretations are often unclear and incautious” (Blüthgen Reference Blüthgen2010: p. 187). The unclear meanings of bipartite network metrics raise the specter of the “file drawer” problem, in which results that are inconclusive, negative, or do not fit with the authors’ agenda are not reported (Fraser et al. Reference Fraser, Parker, Nakagawa, Barnett and Fidler2018). The complexity of bipartite networks makes their analysis subject to these risks in a way that traditional metrics of herbivore damage diversity and intensity are not. A priori decisions about which metrics are most relevant to a given ecological question may address this concern, but Webber et al. (Reference Webber, Schneider and Vander Wal2020) note that the appropriate metric is often unclear for any particular scenario.
Methods
Bipartite networks and several alternative methods were evaluated using existing data, with a focus on the Willershausen assemblage (Adroit et al. Reference Adroit, Girard, Kunzmann, Terral and Wappler2018) as the angiosperm-dominated assemblage with a complete, publicly available dataset that has the highest number of leaves examined. Of the assemblages previously examined in the context of bipartite networks (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021), Willershausen is emphasized as a conservative test, because it is among the few assemblages most likely to have sufficient sampling completeness to quantify host specificity, component communities, and compound communities.
All analyses were performed with R v. 4.1.1 (R Development Core Team 2021). Color schemes were generated with the packages colorbrewer (Neuwirth and Brewer Reference Neuwirth and Brewer2014) and scico (Pedersen and Crameri Reference Pedersen and Crameri2020). For all subsampling routines, we first subsampled each dataset to approximately half of its original size and to progressively smaller sizes, using round numbers when possible for the sake of readability.
Evaluating Bipartite Network Analysis
Sensitivity of Bipartite Network Metrics to Sampling Completeness
The 28 network-level metrics previously used in fossil herbivory studies (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021: table S1; Swain et al. Reference Swain, Azevedo Schmidt, Maccracken, Currano, Dunne, Labandeira and Fagan2021, Reference Swain, Maccracken, Fagan and Labandeira2022: table 1, appendix S4) that are calculated with the networklevel function in the bipartite package (Dormann et al. Reference Dormann, Gruber and Fründ2008) were calculated for the Willershausen assemblage, using subsampling and resampling procedures as sensitivity analyses. Leaves that were not identified to the genus level were removed from the dataset. Each subsampling and resampling routine was iterated 1000 times.
In the first set of routines (“complete”), the entire cleaned Willershausen dataset was analyzed, resampled to the original number of leaves in the cleaned dataset (7333), and subsampled to 3500, 1000, 500, and 300 leaves. Following previous methods (Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022), all host plant taxa represented by fewer than five specimens were removed after the data were resampled or subsampled but before any analyses were performed.
To mirror neontological datasets (Basset et al. Reference Basset, Samuelson and Miller1996; Lewis et al. Reference Lewis, Memmott, Lasalle, Lyal, Whitefoord and Godfray2002) that were recently compared with fossil herbivory data (Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022), we employed a second set of routines (“top 10”) that involved only the 10 host plant taxa at Willershausen with the highest numbers of leaves, ranging from the 948 leaves of Zelkova ungeri Unger, Reference Unger1843 (Kotlaba Reference Kotlaba1963) down to the 164 leaves of Betula maximowicziana Regel, Reference Regel and de Candolle1868. This top 10 dataset of 3602 leaves was resampled to the original number of leaves and subsampled to 1800, 1000, 500, and 300 leaves.
For the sake of comparison, we calculated damage type diversity with coverage-based rarefaction (Chao and Jost Reference Chao and Jost2012) for each resampled and subsampled dataset, using the iNEXT function in the R package iNEXT (Hsieh et al. Reference Hsieh, Ma and Chao2016). We rarefied damage type diversity to the three sample coverage thresholds discussed by Schachat et al. (Reference Schachat, Payne, Boyce and Labandeira2022): 0.7, 0.8, and 0.9.
Bipartite Network Metrics and the Potential for HARKing
To evaluate the possibility of “multiple network metrics that are correlated by variables held in common”—the collinearity among metrics noted as a major pitfall of bipartite network analysis (Webber et al. Reference Webber, Schneider and Vander Wal2020: p. 110)—the same 28 network-level metrics discussed earlier were calculated for a series of fossil assemblages deposited shortly before, during, and after the Paleocene/Eocene thermal maximum (PETM) and the early Eocene climatic optimum (EECO) in the Bighorn Basin and Wind River Basin. Network metrics were calculated after subsampling the data from each assemblage to 300 leaves, following the procedure of Currano et al. (Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021). If a subsample is larger than 50% of the original dataset, the number of possible unique samples decreases. Therefore, subsampling to 300 leaves and generating accurate confidence intervals requires a sample size of at least 600 leaves. The 10 relevant assemblages with 600 or more leaves are Skeleton Coast and Lur'd Leaves from the Bighorn Basin (Wilf et al. Reference Wilf, Labandeira, Johnson and Ellis2006); Dead Platypus, Daiye Spa, Hubble Bubble, the South Fork of Elk Creek, PN, and Fifteenmile Creek from the Bighorn Basin (Currano et al. Reference Currano, Wilf, Wing, Labandeira, Lovelock and Royer2008, Reference Currano, Labandeira and Wilf2010); and the Wind River Interior and Wind River Edge assemblages from the Wind River Basin (Currano et al. Reference Currano, Pinheiro, Buchwaldt, Clyde and Miller2019).
Evaluating Alternatives to Bipartite Network Analysis
Beta Diversity
We evaluated the validity and reliability of measures of abundance gradients (analogous to nestedness: when the damage types observed on one host plant are a subset of the damage types observed on another host plant) and balanced variation in abundance (hereafter “balanced variation”; analogous to turnover: when non-overlapping suites of damage types are observed on different host plants). These are the two components of beta diversity that explicitly account for differences in abundance (Baselga Reference Baselga2017). Our first analysis of beta diversity focuses on the two host plants represented by the highest numbers of leaves at Willershausen: Z. ungeri and Fagus sylvatica Linnaeus, Reference Linnaeus1753. We used each subsampled and resampled dataset generated from the complete Willershausen dataset. Our second analysis of beta diversity focuses on Auritifolia waggoneri and Taeniopteris spp., the two most abundant host plants at Colwell Creek Pond (Schachat et al. Reference Schachat, Labandeira, Gordon, Chaney, Levi, Halthore and Alvarez2014). These two host plants were analyzed at five levels of sampling. They were jointly resampled to the original amount of surface area they comprise in the Colwell Creek Pond dataset (23,527.89 cm2) and were subsampled to a total of 11,750, 8000, 4000, and 2000 cm2. Our third analysis of beta diversity focuses on Macroneuropteris scheuchzeri (Hoffmann, Reference Hoffmann and Keferstein1827) Cleal, Shute & Zodrow, Reference Cleal, Shute and Zodrow1990 and foliage assigned to Sigillariophyllum Grand'Eury, Reference Grand’ Eury1877, the two most abundant host plants at Williamson Drive (Xu et al. Reference Xu, Jin and Labandeira2018). These were jointly resampled to the original number of leaves they comprise in the Williamson Drive dataset (1524) and were subsampled to a total of 750, 600, 450, and 300 leaves. Although surface area measurements were taken for Williamson Drive (Xu et al. Reference Xu, Jin and Labandeira2018), we subsampled these data by number of leaves, because the surface area measurements for individual specimens are not available. Each subsampling routine was iterated 1000 times.
Abundance gradients and balanced variation were calculated for each subsampled and resampled dataset using the beta.pair.abund function in the R package betapart (Baselga and Orme Reference Baselga and Orme2012). We used the coverage function in the R package entropart with the “Chao” estimator (Marcon and Hérault Reference Marcon and Hérault2015) to calculate sample coverage for each of the two plant hosts in each subsampling and resampling routine.
Host Specificity
The sensitivity of host-specificity scores to sampling completeness was evaluated with the complete and top 10 resampling and subsampling routines for the Willershausen dataset. For each set of sampling routines, we recorded the number of host plant taxa on which we observed a randomly selected damage type within the 99th, 74th, and 49th percentiles of prevalence (Table 1).
Table 1. The percentiles of leaves on which damage types were observed at the Willershausen assemblage.

We performed a separate sampling procedure to address the impact of absolute and relative surface area on estimates of host specificity. For this procedure we used the data from Colwell Creek Pond (Schachat et al. Reference Schachat, Labandeira, Gordon, Chaney, Levi, Halthore and Alvarez2014), because this assemblage contains a large amount of surface area examined, and because surface area measurements are available for each individual specimen along with damage type data. We sampled specimens belonging to A. waggoneri, Taeniopteris spp., Evolsonia texana Mamay, Reference Mamay1989, and Supaia thinnfeldioides White, Reference White1929, with replacement, to a series of 51 equally spaced surface area thresholds from 500 cm2 to 25,500 cm2. The smallest of these is approximately 2% of the total surface area, and the largest of these is approximately 100% of the total surface area. We resampled the data to each threshold 10,000 times, for a total of 510,000 iterations. For each iteration, we noted whether DT032 and DT120—which are distributed across all four of these host plant taxa—were restricted to only one host plant, thus falsely appearing to be specialized. If so, we noted the number of specimens on which the damage type had been observed.
Rarefaction of Interactions
The method of Dyer et al. (Reference Dyer, Walla, Greeney, Stireman III and Hazen2010), which measures the diversity of interactions at an assemblage, can be implemented with any algorithm that performs rarefaction. We discuss considerations for coverage-based rarefaction of interactions in the Appendix.
We performed coverage-based rarefaction of interactions on data from Williamson Drive (Xu et al. Reference Xu, Jin and Labandeira2018) and Colwell Creek Pond (Schachat et al. Reference Schachat, Labandeira, Gordon, Chaney, Levi, Halthore and Alvarez2014). We conducted coverage-based rarefaction on the original dataset, and upon iteratively resampling each dataset to the original amount of surface area, and upon subsampling each dataset to 50% and 25% of the original surface area. (Surface area data were collected for each specimen at Williamson Drive but were not published with the damage type data. Therefore, the surface area assigned to each specimen was the mean value for the taxon to which it belongs.) We rarefied each vector of interaction counts to a sample coverage of 0.771, which is the maximum amount of coverage reached by all subsampled datasets.
Because the importance of sampling completeness is a major theme of this contribution, we wished to test the extent to which rarefaction of interactions is robust to incomplete sampling. To understand how rarefaction of interactions might perform on an angiosperm-dominated dataset with complete sampling, we simulated a vector of counts of interactions using the base-R function rlnorm with the settings meanlog=0 and sdlog=1.5. We chose this method because we found that it yielded a distribution of interaction frequencies that closely mirrors that seen at Willershausen. This procedure generated 3000 values, which we had to round to whole integers, because these values represent simulated counts. Upon removing the values that round down to 0, we had 2046 simulated unique interactions that were observed a total of 9597 times. These numbers are approximately double those seen in the Willershausen dataset, so we attributed these simulated interactions to 15,000 leaves, because this is approximately double the number in the Willershausen dataset.
We examined the validity and reliability of rarefaction of interactions in this simulated dataset by subsampling. We subsampled the interactions to one-half of the original count (4798), attributing these to one-half of the original number of leaves (7500). We then subsampled the interactions to one-quarter of the original count (2399), attributing these to one-half of the original number of leaves (3750). We rarefied each vector of subsampled interaction counts to a sample coverage of 0.726, which is the maximum amount of coverage reached by all subsampled datasets.
All rarefaction of interactions was carried out with the estimateD function in the R package iNEXT. All resampling and subsampling procedures were iterated 1000 times.
Results and Discussion
Sensitivity of Bipartite Network Metrics to Sampling Completeness
None of the 28 network-level metrics mentioned in previous studies of fossil herbivory (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021; Swain et al. Reference Swain, Azevedo Schmidt, Maccracken, Currano, Dunne, Labandeira and Fagan2021, Reference Swain, Maccracken, Fagan and Labandeira2022) perform as unbiased estimators for the complete Willershausen dataset (Fig. 3). (An unbiased estimator is an estimator whose average value does not change in response to sampling completeness.) Two simple criteria for robustness to sampling completeness are that the 95% confidence intervals for all subsampling routines contain the mean estimate for the resampling routine and that the 95% confidence interval for the resampling routine contains the mean estimates for all subsampling routines. Coverage-based rarefaction of damage type diversity fulfills these two criteria (Fig. 4), but not a single network metric examined here does.
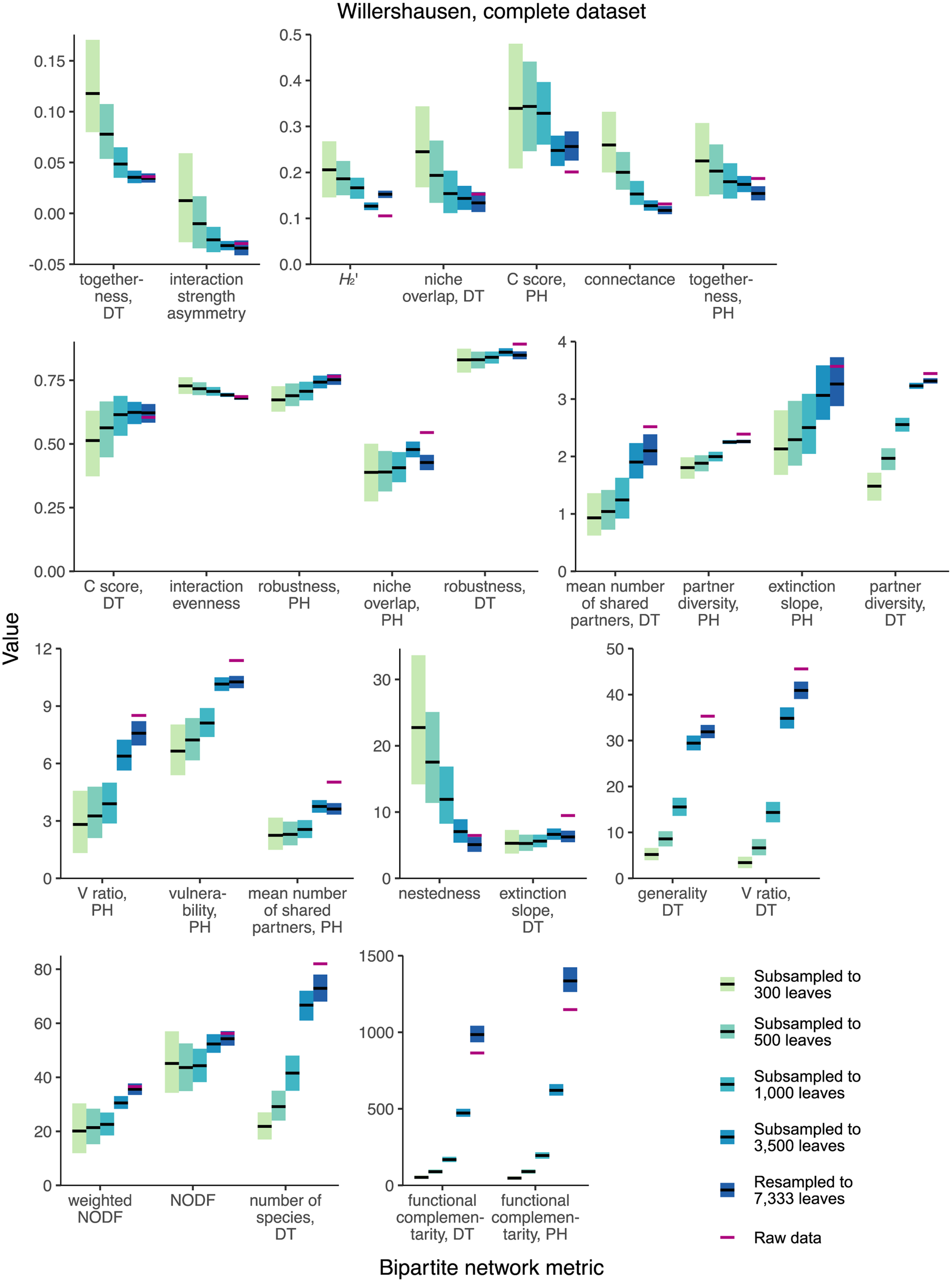
Figure 3. Mean values and 95% confidence intervals for bipartite network metrics generated by resampling and subsampling the cleaned Willershausen dataset in its entirety. DT, damage type; PH, plant host.

Figure 4. An example of a nearly unbiased estimator. Mean values and 95% confidence intervals for coverage-based rarefaction generated by resampling and subsampling the Willershausen dataset. Moreover, coverage-based rarefaction performs as a consistent estimator, in that estimates converge on the true value as sample size increases. No results are presented for 300 subsampled leaves from the complete dataset at sample coverage of 0.9, because some iterations of this sampling routine yielded an observed sample coverage below 0.9.
When the Willershausen data are restricted to only the 10 host plants with the highest number of leaves in the dataset (Fig. 5), 4/28 network metrics fulfill these criteria and thus perform comparably well to coverage-based rarefaction: togetherness for damage types, niche overlap for damage types, C score for damage types, and nestedness.

Figure 5. Mean values and 95% confidence intervals for bipartite network metrics generated by resampling and subsampling data for the 10 host plants at Willershausen represented by the highest numbers of leaves. DT, damage type; PH, plant host.
Only one network metric, C score for damage types, is among the best-performing metrics in both the complete and top 10 analyses of the Willershausen dataset. If the C score for damage types were found to be robust for the majority of available fossil herbivory datasets, which are far less complete than Willershausen, a key question would still need to be answered: What does the C score tell us (Emer et al. Reference Emer, Memmott, Vaughan, Montoya and Tylianakis2016)? The C score has been described in the fossil herbivory literature as “the checkerboard (mutual presence/absence) nature of the interactions” (Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022: p. 243) and as “the randomness of species distribution across an ecosystem” (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021: p. 8), but no outstanding paleontological questions that can be addressed with such a metric have been identified.
Apparent Robustness at Lower Sample Sizes
For many metrics in both the complete and top 10 datasets, the mean estimate and the limits of the confidence intervals change little for the subsampling routines at 1000, 500, and 300 leaves. However, when the resampling routine and the subsampling routines with more than 1000 leaves are taken into account, it is clear that these metrics are biased by sampling incompleteness. The misleading appearance of a lack of bias in certain network metrics seen at lower levels of sampling makes intuitive sense. When a relatively large proportion of realized interactions are unobserved because only 1000 leaves have been sampled, the additional proportion of realized interactions that go unobserved at 500 or 300 leaves will make little difference for various metrics. These findings and this reasoning highlight the danger of evaluating the bias of network metrics by performing sensitivity analyses on smaller datasets. Therefore, any metrics that appear robust to subsampling routines performed on datasets smaller than that of Willershausen should be treated with extreme caution. For these same reasons, methods that quantify the extent to which bipartite network metrics are biased by sampling incompleteness (Swain et al. Reference Swain, Azevedo Schmidt, Maccracken, Currano, Dunne, Labandeira and Fagan2021) may well be unreliable, especially when applied to incomplete datasets.
Implications for Other Assemblages
At any amount of sampling that is realistic for studies of fossil herbivory, the results of bipartite network analysis are biased by sampling completeness. The finding that certain metrics are “relatively robust” (Swain et al. Reference Swain, Azevedo Schmidt, Maccracken, Currano, Dunne, Labandeira and Fagan2021) is an inevitability by chance alone given presentation of dozens of metrics (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021; Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022). Even when we limit our analysis to the 10 most abundant host plants at Willershausen, the mean estimates at 300 and 500 leaves for the best-performing metrics (Swain et al. Reference Swain, Azevedo Schmidt, Maccracken, Currano, Dunne, Labandeira and Fagan2021) either lie beyond (NODF, H2′, connectance, and niche overlap PH) or just barely fall within (niche overlap DT) the 95% confidence interval generated with the resampled dataset. Estimates of these metrics at different sampling intensities are even more discordant for the complete Willershausen dataset.
Paleoecologists have only recently begun to implement Bayesian methods to distinguish true absences of interactions from failures to detect those interactions. In a recent contribution, the authors used a Bayesian framework to estimate the presence or absence of drilling predation on different molluscan species (Smith et al. Reference Smith, Handley and Dietl2022). This allowed the authors to estimate the number of zeros—that is, specimens on which predation was not detected—for which predation was truly absent. Whereas Smith and colleagues were interested in the true prevalence of only one type of feeding trace (drilling predation), fossil herbivory studies often encompass a wide array of traces, as denoted by different damage types. Thus, in fossil herbivory studies, the implementation of a Bayesian frame such as that used by Smith and colleagues would require estimation of the prevalence of a quantity of damage types exceeding the number of specimens from which many plant taxa are known in a given assemblage. For example, at Willershausen, 85 damage types have been observed, and more than 100 host plant taxa are known from only 5–85 specimens. The promise of Bayesian methods is undoubtedly great, and it is not possible to predict the advances that will be seen over the next few decades, but the high ratio of possible damage types to number of specimens per host plant taxon constitutes a major challenge.
Alternatives to Bipartite Networks
Beta Diversity
Our calculations of balanced turnover and abundance gradients for the two dominant host plants at Willershausen show that these metrics are valid and reliable under the resampling routine and under the routine in which the dataset was subsampled to 3500 leaves (Fig. 6A). At lower levels of sampling, the abundance gradient metric yields a similar mean value, but with much wider confidence intervals. The balanced variation metric becomes less valid and reliable at lower levels of sampling. Unsurprisingly, estimates of balanced turnover and abundance gradients are most valid and reliable when coverage is high.

Figure 6. Mean values and 95% confidence intervals for beta-diversity metrics generated by resampling and subsampling data for the two most abundant host plants from (A) Willershausen, (B) Colwell Creek Pond, and (C) Williamson Drive. At the highest sample sizes, represented in dark blue, the data were resampled rather than subsampled. Macronrptrs.scheuchzeri = Macroneuropteris scheuchzeri; Sgllrphyllm. = Sigillariophyllum.
Among the datasets generated by iteratively resampling the Willershausen data and by subsampling the data to 3500 leaves, coverage estimates do not overlap, but estimates of balanced turnover and abundance gradients overlap almost perfectly (Fig. 6A). However, estimates become much less reliable when the Willershausen dataset is subsampled to only 1000 leaves, and the levels of coverage for Zelkova ungeri and Fagus. sylvatica fall to 0.91 and 0.86, respectively.
The Colwell Creek Pond data yield much more valid and reliable results (Fig. 6B). This is perhaps unsurprising, because coverage of the second most-abundant host plant is higher at Colwell Creek Pond than at Willershausen. Whereas it is very rare for two host plants within a single assemblage to have such high sample coverage—0.990 for Auritifolia waggoneri and 0.989 for Taeniopteris spp.—our findings suggest that valid and reliable estimates of balanced turnover and abundance gradients are achievable for those rare assemblages with two host plants that are nearly completely sampled.
The Williamson Drive data yield results that are even more valid and reliable than those for Colwell Creek Pond (Fig. 6C). This is a bit surprising: although the most dominant host plant at Williamson Drive, Macroneuropteris scheuchzeri, has sample coverage of 0.991, the second most-dominant host plant, Sigillariophyllum foliage, has sample coverage of only 0.948. This is quite a bit less than that of Taeniopteris spp. at Colwell Creek Pond, and we do not yet have enough paleontological data to evaluate the consequences of this reduced sample coverage. For Williamson Drive, balanced variation and abundance gradients essentially perform as unbiased and consistent estimators to nearly the same extent as does coverage-based rarefaction (Fig. 4). Further analyses are needed to determine exactly why these two metrics perform somewhat better for the Paleozoic data than for Willershausen—richness of damage types may be a key determinant—and particularly why these metrics perform better for Williamson Drive than for Colwell Creek Pond.
Nevertheless, it is clear that these two components of beta diversity are a preferable alternative to bipartite network metrics. They are more valid and reliable than nearly any bipartite network metric that has been examined for fossil herbivory (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021; Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022). Their meanings are clear, as is the difference between them. They can be calculated for pairwise comparisons among host plants, or can be used to generate a single value for an entire assemblage (Baselga and Orme Reference Baselga and Orme2012; Baselga Reference Baselga2017), and can thus be used whether an assemblage contains 2 or 20 host plants with nearly complete sampling.
Host Specificity
The results of our resampling and subsampling procedures demonstrate that the traditional method for assigning host-specificity scores is strongly biased by sampling completeness: at lower levels of sampling, the host breadth of a damage type inevitably decreases (Fig. 7). For example, in the Colwell Creek Pond resampling routines, we treated each iteration in which the generalist DT032 or DT120 damage type was restricted to only one host plant taxon as a false positive finding of specialization. DT032 appeared on only one host plant taxon in 2.72% of iterations; DT120, in 3.78%. When a finding of specialization requires a damage type to appear on three or more specimens, following the convention established by Wilf and Labandeira (Reference Wilf and Labandeira1999), the false positive rate falls to 0.93% for DT032 but remains at 3.34% for DT120.
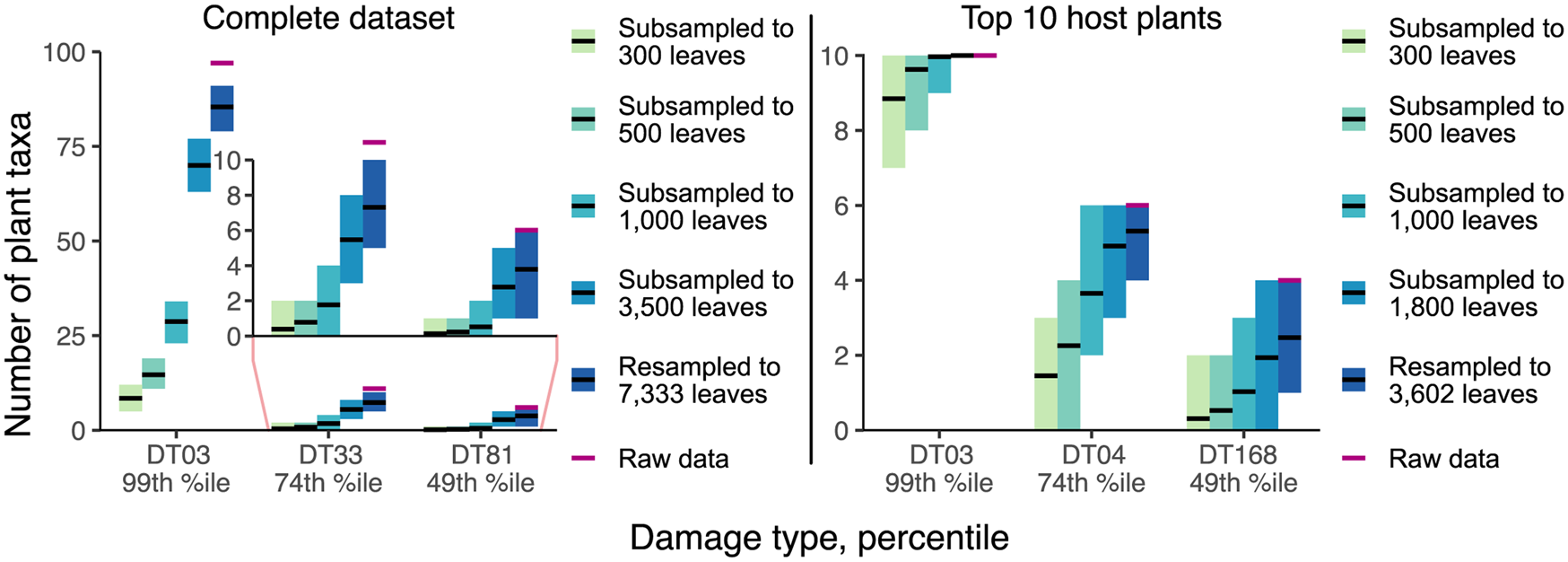
Figure 7. Mean values and 95% confidence intervals for the number of plant taxa on which various damage types appear, calculated with the Willershausen dataset.
The inadequacy of the three-specimen threshold for designation of a damage type as “specialized” is shown by the frequencies of false positive results (Fig. 8). These frequencies appear to follow lognormal distributions. For DT032, which was observed on fewer leaves than DT120, σ > 1 such that the greatest proportion of false positive results occur when this damage type is observed on only one specimen. However, for DT120, σ < 1 such that 4.7% of false positive results occur when this damage type is observed on only one specimen, 8.7% occur when this damage type is observed on four specimens, and 4.9% occur when this damage type is observed on nine specimens. Thus, the three-specimen threshold protects against only a small fraction of false positives.
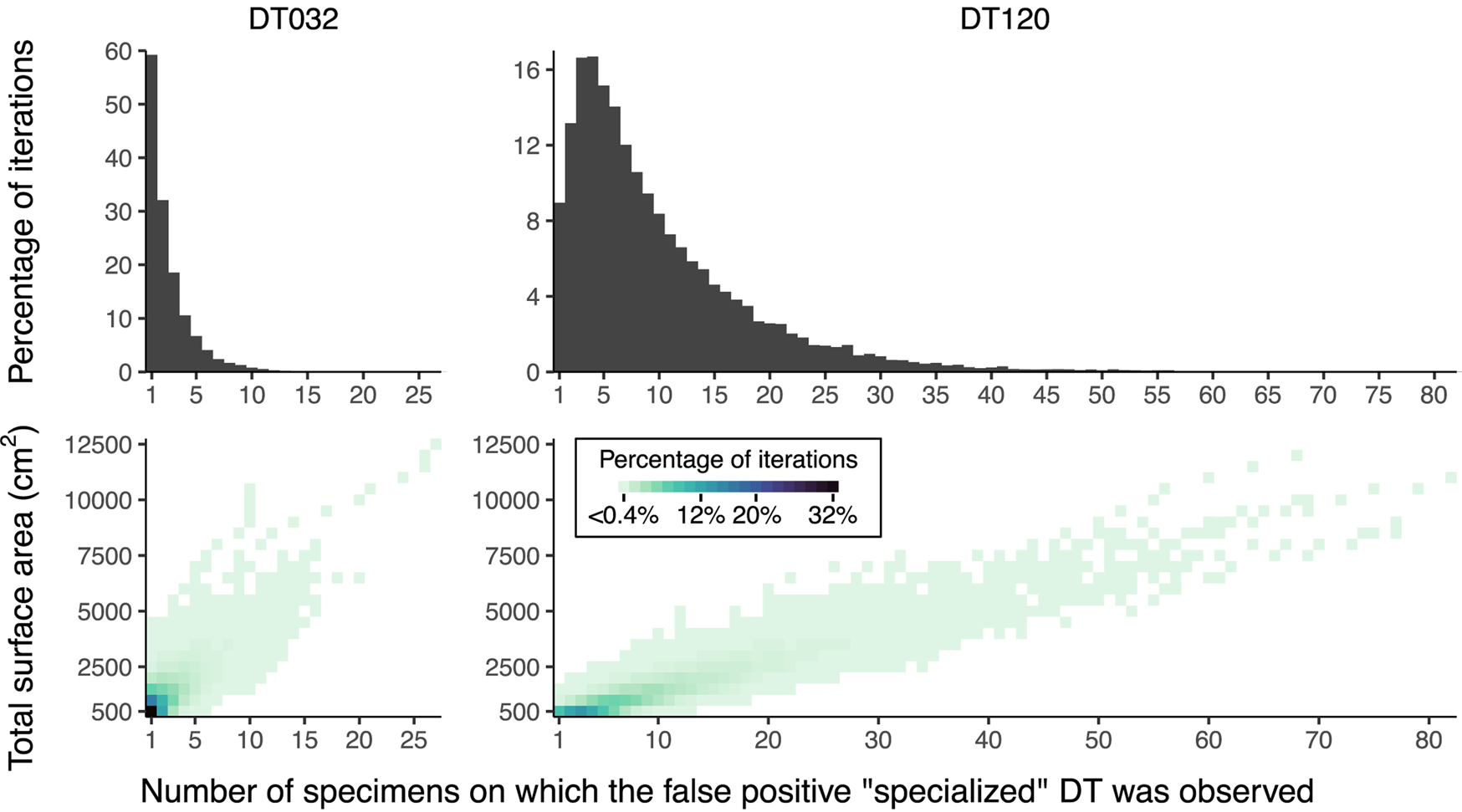
Figure 8. False positive results of “specialized” damage generated by iteratively resampling data from Colwell Creek Pond. We treated each iteration in which DT032 or DT120 was observed on only one host plant taxon as a false positive. The heat maps show the percentage of iterations for each amount of subsampled surface area in which a false positive result was recovered, arranged by the number of specimens on which the damage type was observed. The histograms show the summed percentages, by number of specimens.
Rarefaction of Interactions
Coverage-based rarefaction of interactions performs as an unbiased and consistent estimator: as sampling completeness decreases, the mean estimate changes negligibly while confidence intervals widen (Fig. 9). Resampled estimates and confidence intervals are often invalid for rarefaction of interactions, because the number of singletons in a resampled dataset tends not to exceed the number of singletons in the original dataset. The number of singletons is one of the main determinants of estimated sample coverage; thus, resampled datasets tend to have higher estimated coverage than the original datasets. This means that coverage-based rarefaction will generate lower estimates for resampled data than for subsampled data. This is abundantly clear for rarefaction of interactions in the simulated dataset and is also quite notable for Williamson Drive. The estimation of confidence limits from iteratively sampled data should therefore be performed with subsampled, rather than resampled, data whenever the mean estimate generated with resampled data is clearly invalid. The methodology of coverage-based rarefaction of interactions is illustrated in Figure 10.

Figure 9. Mean values and 95% confidence intervals for coverage-based rarefaction of interactions. The datasets presented here are Williamson Drive and Colwell Creek Pond, both from the Permian of Texas (rarefied to a sample coverage of 0.771) and a simulated dataset that mimics the patterns seen among angiosperms at Willershausen (rarefied to a sample coverage of 0.726).
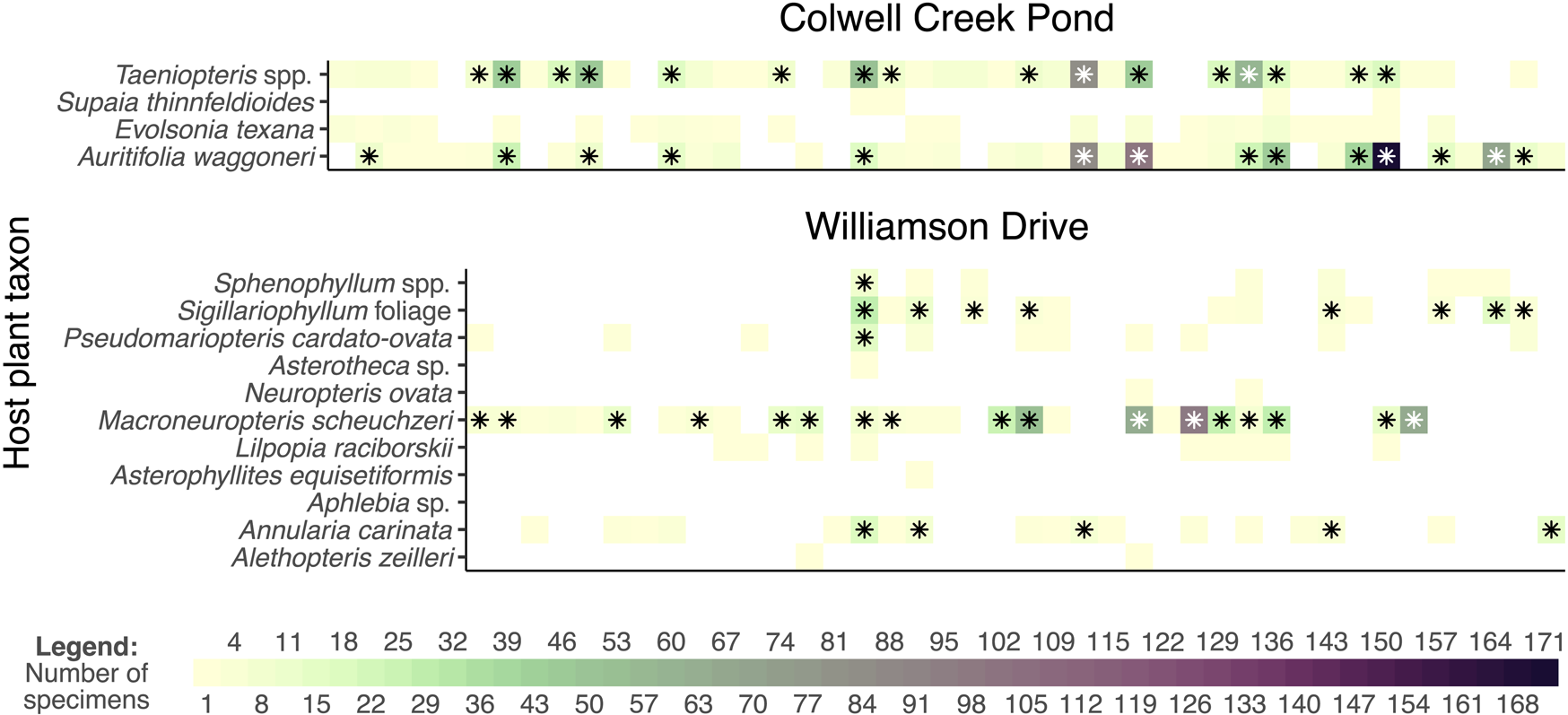
Figure 10. Comparison of the raw and rarefied interaction data from Colwell Creek Pond and Williamson Drive. Each column of each graph represents a damage type. The heat maps show the prevalence of each interaction, and the asterisks denote interactions that remain after rarefying data from each assemblage to a sample coverage of 0.771.
An Example of Bipartite Network Metrics and the Potential for Metric Hacking
While it has been argued that bipartite network metrics allow a more finely resolved, “in-depth” understanding of the relationships between host plants and damage types (Swain et al. Reference Swain, Azevedo Schmidt, Maccracken, Currano, Dunne, Labandeira and Fagan2021), others argue that the multiple comparisons presented in many network studies often contain spurious results (Webber et al. Reference Webber, Schneider and Vander Wal2020). To evaluate which of these two views of multiple comparisons in network studies is applicable to fossil herbivory datasets, we calculated bipartite network metrics for one of the most iconic and intensely studied series of assemblages in this discipline: Paleocene and Eocene floras of the western interior of North America (Wilf and Labandeira Reference Wilf and Labandeira1999; Currano et al. Reference Currano, Wilf, Wing, Labandeira, Lovelock and Royer2008, Reference Currano, Labandeira and Wilf2010). The finding of increased insect herbivory at the PETM is supported by quantitative measures of herbivorized leaf area (Currano et al. Reference Currano, Laker, Flynn, Fogt, Stradtman and Wing2016) and by damage type diversity, whether rarefied by number of leaves (Currano et al. Reference Currano, Labandeira and Wilf2010)—an older practice shown to be biased by differences in leaf surface area among host plant taxa (Schachat et al. Reference Schachat, Labandeira and Maccracken2018)—or rarefied by sample coverage (Schachat et al. Reference Schachat, Payne, Boyce and Labandeira2022). Changes in herbivory at the EECO have not been examined as thoroughly (Currano et al. Reference Currano, Pinheiro, Buchwaldt, Clyde and Miller2019), but the logic about climate, nutrient availability, and herbivory used to describe the PETM (Currano et al. Reference Currano, Wilf, Wing, Labandeira, Lovelock and Royer2008, Reference Currano, Labandeira and Wilf2010) ought to apply to the EECO as well.
When the 28 bipartite network metrics considered here are calculated for the Paleocene–Eocene assemblages of the Bighorn Basin and Wind River Basin (Fig. 11), none of these metrics yield extreme values for the PETM Hubble Bubble assemblage (Currano et al. Reference Currano, Wilf, Wing, Labandeira, Lovelock and Royer2008) or the EECO Wind River Interior assemblage (Currano et al. Reference Currano, Pinheiro, Buchwaldt, Clyde and Miller2019). If these metrics are taken at face value, rather than being dismissed due to their susceptibility to sampling bias, they suggest that extreme climate change does not have a perceptible impact on plant–insect interactions. For a variety of metrics (interaction strength asymmetry, the C score for host plants, connectance, togetherness, partner diversity for damage types, generality for damage types), it is not the assemblage deposited during the PETM, but the assemblage deposited just afterward, that yields the most extreme values. This assemblage, South Fork of Elk Creek, was immediately noted for having only two host plants preserved in meaningful quantities (Currano et al. Reference Currano, Wilf, Wing, Labandeira, Lovelock and Royer2008; Currano, Reference Currano2009): a peculiarity that has not been ascribed with ecological significance (Currano et al. Reference Currano, Wilf, Wing, Labandeira, Lovelock and Royer2008, Reference Currano, Labandeira and Wilf2010; Currano Reference Currano2009). However, this long-known peculiarity appears to be driving temporal patterns in approximately one-quarter of bipartite network metrics. (For all other assemblages shown in Fig. 11, the mean number of host plant taxa in each subsampling iteration ranges from 4.7 to 11.9.)
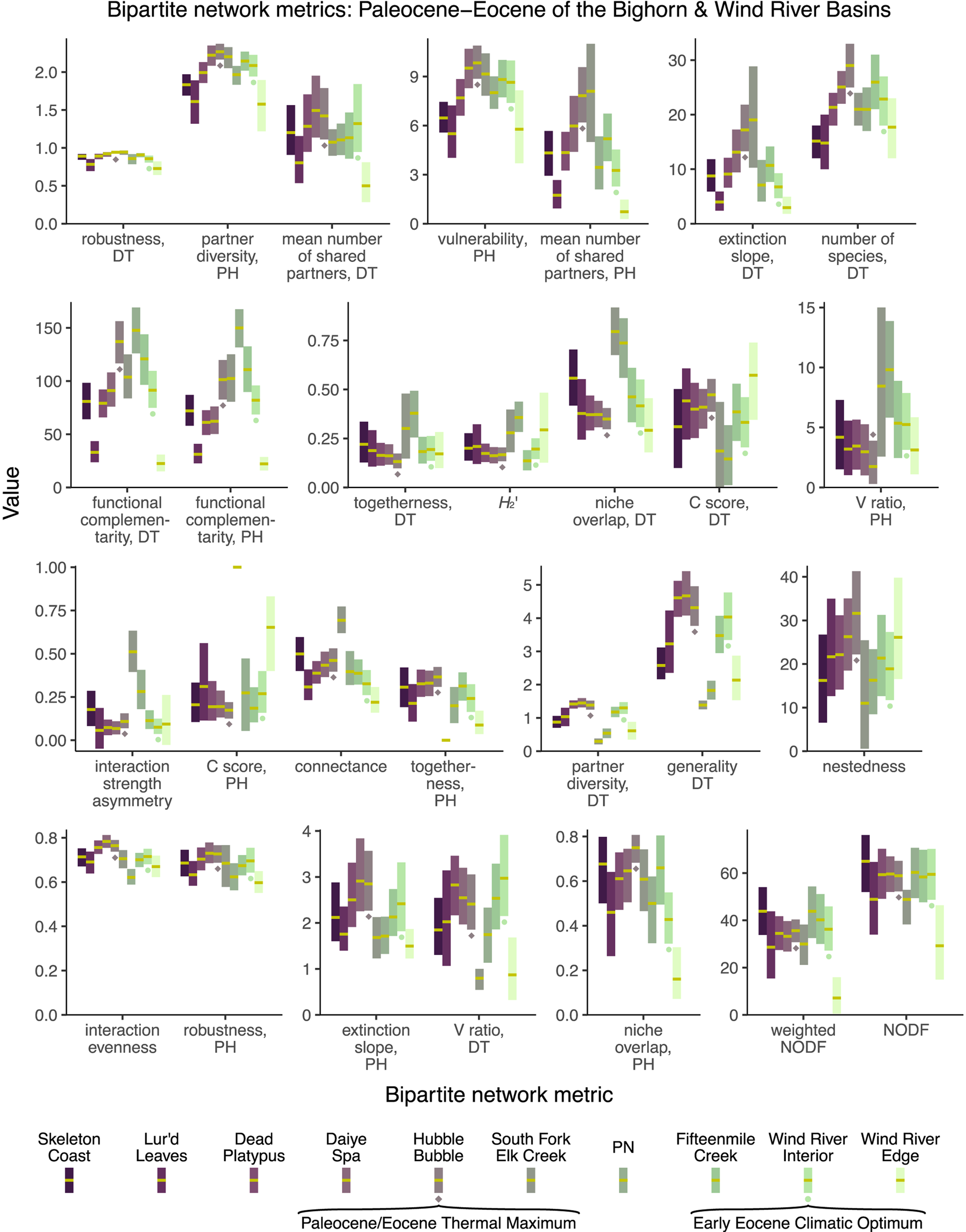
Figure 11. Mean values and 95% confidence intervals for bipartite network metrics, generated by subsampling each dataset to 300 leaves. DT, damage type; PH, plant host.
Different combinations of these metrics support different narratives. Of the 28 bipartite network metrics, approximately one-third suggest that the PETM and EECO had dissimilar impacts on the relationship between host plants and damage types, approximately one-third suggest that the PETM and EECO had similar impacts, and approximately one-third yield inconclusive results (Fig. 11, Table 2). The PETM itself yields a variety of possible conclusions. More than two-thirds of these metrics suggest that the relationship between host plants and damage types did not drastically change from the very late Paleocene to the PETM, and less than one-quarter are inconclusive (Fig. 11, Table 2). The only two metrics that suggest a drastic change in the relationship between host plants and damage types at the PETM—functional complementarity for host plants and for damage types—are the two metrics that show the greatest amount of spread overall (Figs. 3, 5, 11). Moving from the PETM into the Eocene, more than one-quarter of these metrics suggest that the relationship between host plants and damage types did not change from the PETM to its immediate aftermath, more than one-third suggest that this relationship did indeed change, and more than one-quarter are inconclusive (Fig. 11, Table 2).
Table 2. The variety of narratives about the PETM supported by different combinations of bipartite network metrics.
The only metric that returns a more extreme value for the PETM than for the two assemblages that immediately predate and postdate it—that is, the mean value for the PETM lies beyond the 95% confidence intervals for any of these four other assemblages—is “number of species, DT.” We have presented this metric here as if it were a bipartite network metric, because it was previously reported as such (Currano et al. Reference Currano, Azevedo-Schmidt, Maccracken and Swain2021; Swain et al. Reference Swain, Maccracken, Fagan and Labandeira2022), and because it is calculated with the networklevel function in the bipartite package in R (Dormann et al. Reference Dormann, Gruber and Fründ2008). However, this is not truly a bipartite network property, in that it does not respond to the distribution of damage types among the host plants.
Bipartite network properties fail to identify the PETM as an anomaly. This finding necessitates a reckoning as to whether bipartite network analysis provides additional nuance and context to traditional metrics such as the herbivory index and rarefied damage type diversity, or alternatively, whether these metrics are too biased at realistic sample sizes to provide results that warrant interpretation. If the canonical notion of uniquely intense and diverse insect herbivory at the PETM is erroneous, that notion should of course be challenged. But, for the many reasons detailed earlier, the various narratives that emerge from bipartite network analysis that contradict the accepted influence of the PETM on insect herbivory are quite likely artifacts of sampling incompleteness and unevenness.
Conclusions
The challenge of linking host plants to damage types through bipartite network analysis is threefold. First, sampling incompleteness does not simply cause increased uncertainty, as is the case for consistent and unbiased estimators such as the herbivory index or coverage-based rarefaction of damage type diversity; instead, sampling incompleteness typically leads to inaccurate, misleading results. Second, the wide variety of bipartite network metrics creates many opportunities for HARKing. Those opportunities are exacerbated by the unclear meanings of these metrics. And third, many damage types violate both the taxonomic species concept and the trophic species concept, depriving specialization of its ecological meaning in this context.
No amount of sampling completeness can remove the potential for HARKing presented by bipartite network analysis, but our results show that alternative methods that are insusceptible to HARKing can be used to evaluate host specificity, to compare component communities, and to measure the diversity of interactions at an assemblage. Rarefied interaction richness and the components of beta diversity are much more likely than bipartite network metrics to perform as unbiased and consistent estimators and do not require complete sampling of damage types across all host plants at an assemblage. Much essential information is still lacking: the exact sample coverage required for valid measurement of abundance gradients, balanced variation, and the diversity of interactions; as well as the surface area data required for evaluation of host specificity, which are unavailable for most published assemblages. However, the first step is understanding which analyses are meaningful and which measurements are needed for those analyses to be valid.
At present, there are a number of large gaps in our knowledge of fossil herbivory. First is the nearly complete lack of Pennsylvanian or Jurassic assemblages examined for herbivory and the lack of early to mid-Cretaceous assemblages. Second is the general lack of assemblages examined from tropical latitudes. Third is the widespread lack of surface area measurements, which are necessary for evaluating the intensity of herbivory (Schachat et al. Reference Schachat, Labandeira and Maccracken2018). Fourth is the widespread lack of counts of the number of times that each damage type appears on each leaf, termed “feeding event occurrences.” These data can be used to evaluate various hypotheses about the causes of increased herbivory (Schachat et al. Reference Schachat, Payne, Boyce and Labandeira2022). In light of the limited amount of time that paleontologists are able to spend collecting fossil herbivory data, we believe that addressing these four gaps is the most important use of investigator effort.
Acknowledgments
We thank three reviewers who provided helpful feedback that improved our article. We thank Conrad Labandeira for extensive feedback. We thank all researchers who have collected fossil herbivory data, especially Benjamin Adroit and colleagues who collected and shared the Willershausen dataset.
Declaration of Competing Interests
The authors declare no competing interests.
Appendix
Calculating p-Values for Host Specificity
The absolute amount of surface area examined should be taken into account when determining host specificity, because if the total amount of surface area is very small, the apparent restriction of a damage type to a particular clade of host plants will very possibly be an artifact of insufficient sampling. The relative amount of surface area should be taken into account, because this determines the probability that a damage type would falsely appear to be restricted to a particular clade of host plants.
Consider a hypothetical assemblage in which 100,000 cm2 of surface area has been examined. If DT001 is restricted to a clade of host plants represented by a mere 500 cm2 of surface area, and if DT001 is found on all 15 specimens belonging to the clade at this assemblage, then DT001 indeed appears to be specialized. This finding is supported by the large amount of surface area examined, by the moderately high number of specimens on which DT001 has been found, and by the small amount of relative surface area belonging to the plant clade in question, which confers a low probability that all detected incidents of DT001 would be restricted to this clade due to chance alone.
However, at Colwell Creek Pond, the host plant Auritifolia waggoneri accounts for greater than 60% of the broadleaf surface area examined. Therefore, especially if the total amount of surface area examined is low, a generalized damage type may appear to be restricted to A. waggoneri due to chance alone—particularly if the damage type is observed on only a few specimens. To test the frequency with which this sort of false positive finding of specialized herbivory may occur, we resampled the data from Colwell Creek Pond for the four host plant taxa from this assemblage that unambiguously meet the criteria for inclusion outlined by Swain et al. (Reference Swain, Azevedo Schmidt, Maccracken, Currano, Dunne, Labandeira and Fagan2021): A. waggoneri (63% of total broadleaf surface area), Taeniopteris spp. (28%), Evolsonia texana (9%), and Supaia thinnfeldioides (1%). Our analysis focuses on two damage types, DT032 and DT120. Both of these damage types occur on all four of these host plants, with distributions that approximate the amount of surface area examined for each host plant: the majority of incidences of each damage type are on A. waggoneri (63%–89%), followed by Taeniopteris spp. (10%–25%), E. texana (1%–10%), and, finally, S. thinnfeldioides (1%–3%).
When a damage type is observed only on one clade of host plants at an assemblage, the surface area of those host plants can be used to test the null hypothesis that the damage type is restricted to a certain plant clade simply by chance. The proportion of all surface area examined at the assemblage that belongs to the clade in question—whether it is a genus or species, implying specialized host specificity, or a higher clade, implying intermediate specificity—can be raised to the number of specimens on which the damage type was observed. This process generates a p-value that can be used to test the null hypothesis of generalized host specificity. Consider an example in which a damage type appears to have an intermediate host specificity because it occurs only on plants belonging to the same order. If this order accounts for 40% of all surface area examined at the assemblage, and if the damage type has been observed on five specimens, the p-value for its host specificity is 0.45 = 0.01024. This value is below 0.05, and thus the damage type has been observed on enough specimens to reject the null hypothesis of generalized host specificity. However, a correction for multiple comparisons, such as the Bonferroni correction or the Benjamini–Hochberg correction, should be used if this procedure is carried out for more than one damage type.
These findings presented in our “Results and Discussion” section suggest that the more conservative Bonferroni correction should be used instead of the Benjamini–Hochberg correction when host-specificity p-values are calculated for multiple damage types. Surface area data from additional assemblages, with as much area as Colwell Creek Pond or more, are needed to determine whether the Benjamini–Hochberg correction will suffice.
Another fundamental, unresolved issue pertaining to the assignment of host-specificity scores is the definition of “specialized” and “intermediate” host specialization. If a damage type occurs on multiple genera within the same family, is it a specialized damage type, because it is restricted to one family, or is it an intermediate damage type, because it occurs on multiple genera? To our knowledge, this question has never been answered, leaving each team of authors to draw the boundaries between specialized, intermediate, and generalized host specificity wherever they please. To our knowledge, the locations of these boundaries are not typically articulated in publications, leading to a lack of reproducibility. Because the majority of herbivorous insects feed on plants belonging to a single family (Forister et al. Reference Forister, Novotny, Panorska, Baje, Basset, Butterill, Cizek, Coley, Dem, Diniz, Drozd, Fox, Glassmire, Hazen, Hrcek, Jahner, Kaman, Kozubowski, a Kursar, Lewis, Lill, Marquis, Miller, Morais, Murakami, Nickel, a Pardikes, Ricklefs, Singer, Smilanich, Stireman, Villamarín-Cortez, Vodka, Volf, Wagner, Walla, Weiblen and Dyer2015), we recommend that a damage type that occurs on a single family be considered “specialized” and that a damage type that occurs on multiple families within a single order be considered “intermediate.”
We do not advocate assigning host-specificity scores to damage types. For reasons outlined in the “Introduction,” specialist herbivores can be largely or entirely responsible for a “generalized” damage type. For reasons outlined in the “Results and Discussion,” a “generalized” damage type can appear to be “specialized” due to sampling incompleteness. However, should any research teams continue to assign host-specificity scores, our method for generating p-values protects against false positive findings of specialized herbivory, and our recommended boundaries between specialized, intermediate, and generalized host specificity provide an objective, reproducible, working definition.
Considerations for Coverage-based Rarefaction of Interactions
The input used for bipartite network analysis and for rarefaction of interactions is essentially the same (Table A1). Bipartite network analysis uses a matrix in which each row represents a host plant, each column represents a herbivore (or, for fossil herbivory, a damage type), and each cell represents the number of times that a given interaction was observed. In the example shown in Table A1, DT001 was observed on one specimen belonging to plant sp. 1 and DT002 was observed on five specimens belonging to plant sp. 1. For rarefaction of interactions, the matrix is vectorized, or transformed into a single row. The information about particular host plants and damage types is removed, only the numbers of observations remain, the ordering of these observations does not matter, and it does not matter whether unobserved interactions with a value of 0 are retained in the vector.
Table A1. A toy example of the input used for bipartite network analysis. For rarefaction of interactions (Dyer et al. Reference Dyer, Walla, Greeney, Stireman III and Hazen2010), the input would be a vectorized version of this matrix, which could take any of the following forms: [ 1 5 0 2 2 0 0 6 0 0 1 0 0 1 0 1 0 3 0 1 ], or [ 1 5 2 2 6 1 1 1 3 1 ], or [ 6 5 3 2 2 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 ], or [ 6 5 3 2 2 1 1 1 1 1 ].

This vector is then used for a subsampling procedure and can be subsampled to a threshold of sample coverage, as Schachat et al. (Reference Schachat, Payne, Boyce and Labandeira2022) have advocated. Whereas bipartite network analysis produces misleading results with incomplete sampling by treating rare, undetected interactions as true absences, rarefaction of interactions subsamples the observed interactions such that the rare, undetected interactions are removed from the dataset and thus cannot bias the results. Once the dataset for an assemblage reaches the coverage threshold to which all assemblages are subsampled, additional sampling completeness—revisiting an assemblage that already reaches a sample coverage of 0.9 and collecting additional data until sample coverage reaches 0.95—will not change the results on average, in contrast to bipartite network analysis. This is because the progression of an unbiased sampling routine will lead to additional observations of common interactions while allowing the observation of new, rare interactions.
In a typical rarefaction analysis in the context of fossil herbivory, the input is a vector that contains the number of specimens upon which each damage type has been observed. For example, if DT001 and DT002 have each been observed on three specimens and DT003 has been observed on one specimen, the input vector would take the form of [3 3 1]. To rarefy the interactions rather than the damage type incidences in this toy example, if DT001 was observed on three specimens belonging to the same plant host and DT002 was observed on two different plant hosts, the input vector would take the form of [3 2 1 1]: the second “3” in the original vector, corresponding to DT002, has been split into a “2”, representing two incidences of this damage type on one plant host, and a “1”, representing an incidence of this same damage type on a different plant host.
There is a computational issue with increasing the number of values in an input vector that equal 1: this reduces sample coverage (Good Reference Good1953). Because scaling rarefaction curves by the number of leaves examined is an inadequate substitute for scaling by the amount of surface area examined (Schachat et al. Reference Schachat, Labandeira and Maccracken2018), coverage-based rarefaction is the only appropriate method for comparing assemblages that lack measurements of surface area. But the sampling completeness that is needed to rarefy damage type diversity (Schachat et al. Reference Schachat, Payne, Boyce and Labandeira2022) will far fall short of the sampling completeness needed to rarefy the diversity of interactions. For example, when we iteratively subsampled the Willershausen dataset to 1000 leaves, sample coverage was as low as 0.599—a level at which comparisons will be grossly underpowered, as discussed by Schachat et al. (Reference Schachat, Payne, Boyce and Labandeira2022). Therefore, we evaluated rarefaction of interactions with a simulated dataset.
Host Plants with Sample Coverage above 0.99
The following is a nonexhaustive list of host plants censused for fossil herbivory, for which sample coverage is above 0.99. Zelkova ungeri from Willershausen (Adroit et al. Reference Adroit, Girard, Kunzmann, Terral and Wappler2018); Macginitiea gracilis Lesquereux, Reference Lesquereux and Hayden1872 (Wolfe and Wehr Reference Wolfe and Wehr1987) from PN (Currano et al. Reference Currano, Labandeira and Wilf2010); Heidiphyllum elongatum Morris, Reference Morris and de Strzelecki1845 (Retallack Reference Retallack1981) from Aasvoëlberg 311 (Labandeira et al. Reference Labandeira, Anderson, Anderson and Tanner2018); Sphenobaiera schenckii Feistmantel, Reference Feistmantel1886 (Florin Reference Florin1936) from Birds River 111 (Labandeira et al. Reference Labandeira, Anderson, Anderson and Tanner2018); Platanus raynoldsii Newberry, Reference Newberry1868 from Mexican Hat (Wilf et al. Reference Wilf, Labandeira, Johnson and Ellis2006; Donovan et al. Reference Donovan, Wilf, Labandeira, Johnson and Peppe2014); Macroneuropteris scheuchzeri from Williamson Drive (Xu et al. Reference Xu, Jin and Labandeira2018); A. waggoneri from Colwell Creek Pond (Schachat et al. Reference Schachat, Labandeira, Gordon, Chaney, Levi, Halthore and Alvarez2014); Quercus sp. Linnaeus, Reference Linnaeus1753 from Longmen (Su et al. Reference Su, Adams, Wappler, Huang, Jacques, Liu and Zhou2015).
When coverage equals 1, this is typically misleading, as it most likely signifies that either no damage has been found on the host plant taxon in question (the coverage function in the entropart package calculates coverage of 1 when there is no damage) or that the sample size is much too small, which can spuriously lead to no singleton damage types. For example, Fabaceae sp. WW042 at the PN assemblage (Currano et al. Reference Currano, Labandeira and Wilf2010) is represented by 16 leaves. Three damage types are observed: DT002 is on two leaves, DT012 is on six leaves, and DT032 is on two leaves. Coverage equals 1. However, if the number of leaves with DT002 is experimentally reduced from two to one, coverage falls from 1 to 0.9111. The only host plant we are aware of for which coverage of 1 is not a spurious artifact is Quercus sp. from Longmen (Su et al. Reference Su, Adams, Wappler, Huang, Jacques, Liu and Zhou2015). Twelve damage types were found on the 1027 leaves examined. All damage types were found on at least five leaves. If the number of leaf specimens with DT045 is experimentally reduced from five to four, coverage remains at 1. This suggests a rule of thumb for determining whether a high coverage estimate is an artifact: if coverage remains above 0.99 after one leaf specimen with the rarest non-singleton damage type is experimentally removed from the dataset, the coverage estimate is indeed robust. Notably, when we subsampled the Willershausen dataset to at least 1000 leaves and iterated this procedure 10,000 times, coverage never exceeded 0.9972. It therefore appears that all coverage estimates that equal 1 would become slightly lower—if not far lower—with additional sampling. Thus, a coverage estimate of 0.995 is a stronger indicator of complete sampling than is a coverage estimate of 1.



















