Introduction
Twin pregnancies are associated with a higher rate of adverse outcomes compared to singleton pregnancies. They are frequently complicated by preterm delivery, intrauterine growth restriction, and Neonatal Intensive Care Unit (NICU) or Special Care Nursery (SCN) admission (Luke & Brown, Reference Luke and Brown2006). It has been suggested that the increased risk of adverse outcomes related to twin pregnancies could be exacerbated with concomitant gestational diabetes mellitus (GDM; Rauh-Hain et al., Reference Rauh-Hain, Rana, Tamez, Wang, Cohen, Cohen and Thadhani2009; Simoes et al., Reference Simoes, Queiros, Correia, Rocha, Dias and Blickstein2011). However, studies investigating the consequences of GDM in twin pregnancies are small and present conflicting evidence, with some finding no difference in perinatal outcomes between GDM and non-GDM twins (Guillen et al., Reference Guillen, Herranz, Barquiel, Hillman, Burgos and Pallardo2014; Moses et al., Reference Moses, Webb, Lucas and Davis2003) and others even demonstrating better outcomes (Luo et al., Reference Luo, Simonet, Wei, Xu, Rey and Fraser2011; Okby et al., Reference Okby, Weintraub, Sergienko and Eyal2014). More recent literature highlights the possible adverse effects of strict glycemic control of GDM in twin pregnancies (Foeller et al., Reference Foeller, Zhao, Szabo and Cruz2015; Fox et al., Reference Fox, Gerber, Saltzman, Gupta, Fishman, Klauser and Rebarber2016; Klein et al. Reference Klein, Mailath-Pokorny, Leipold, Krampl-Bettelheim and Worda2010; Luo et al., Reference Luo, Simonet, Wei, Xu, Rey and Fraser2011; Moses et al., Reference Moses, Webb, Lucas and Davis2003; Okby et al., Reference Okby, Weintraub, Sergienko and Eyal2014), although this is not universally reported (Cho et al., Reference Cho, Shin, Yang, Ryu, Kim, Han and Choi2006; Gonzalez Gonzalez et al., Reference Gonzalez Gonzalez, Goya, Bellart, Lopez, Sancho, Mozas and Bartha2012; Guillen et al., Reference Guillen, Herranz, Barquiel, Hillman, Burgos and Pallardo2014; Rauh-Hain et al., Reference Rauh-Hain, Rana, Tamez, Wang, Cohen, Cohen and Thadhani2009; Simoes et al., Reference Simoes, Queiros, Correia, Rocha, Dias and Blickstein2011).
The current guidelines used for GDM diagnosis and management for twin pregnancies are based on limited knowledge of the effect of hyperglycemia in this cohort. The two landmark randomised controlled trials (RCTs) evaluating screening and treatment of GDM either did not include twin pregnancies (Landon et al., Reference Landon, Spong, Thom, Carpenter, Ramin, Casey and Anderson2009), or only included a small number of twins and did not separate the data during analysis (Crowther et al., Reference Crowther, Hiller, Moss, McPhee, Jeffries and Robinson2005). Despite this, the diagnostic criteria for GDM endorsed by the International Association of Diabetes and Pregnancy Study Group (IADPSG), World Health Organization, and Australasian Diabetes in Pregnancy Society (ADIPS) are recommended to be applied to all pregnancies, including twins (Metzger et al., Reference Metzger, Gabbe, Persson, Buchanan, Catalano, Damm and Schmidt2010; Nankeris et al., Reference Nankeris, McIntyre, Moses, Ross, Callaway, Porter and McElduff2014; WHO Guidelines Approved by the Guidelines Review Committee, 2013). Furthermore, the blood glucose targets for ongoing management of GDM are all based on studies carried out on singleton pregnancies (Crowther et al., Reference Crowther, Hiller, Moss, McPhee, Jeffries and Robinson2005).
Given the limited evidence examining twins and GDM under the most recent ADIPS criteria, it is timely to investigate whether GDM adds further risk to an inherently high-risk pregnancy. We examined the type and magnitude of risk incurred by twin pregnancy and by a diagnosis of GDM separately to ascertain their relative associations with poor perinatal outcomes.
Materials and Methods
A retrospective audit was conducted on the outcomes of all women who delivered at the Royal Women’s Hospital, Melbourne, between January 2016 and December 2017. Pregnancies complicated by pregestational diabetes (i.e., diabetes diagnosed prior to pregnancy, type 1 or type 2 diabetes mellitus), congenital anomalies, delivery before 22 weeks’ gestation, or fet al death in utero were excluded. Data were collected prospectively by the institutional Quality and Safety Unit from the Maternity Care Information System (MCIS, GE Healthcare, Little Chanfont, United Kingdom) and collated in MS Excel spreadsheets (Microsoft, Redmond, USA). Data were analyzed retrospectively through selecting the demographics and outcomes of interest. Demographics and background information examined were age, ethnicity, body mass index (BMI), artificial reproductive technology, previous cesarean delivery, chorionicity (in twin pregnancy), and smoking status. The results of the Oral Glucose Tolerance Test (OGTT) test for each patient (i.e., fasting, 1-h and 2-h values) were also recorded. Based on ADIPS recommendations, gestational diabetes was diagnosed after a fasting 75 g Glucose Tolerance Test at any time in pregnancy with one or more of the following blood glucose ranges: fasting: 5.1–6.9 mmol/L; 1 h: >10.0 mmol/L; 2 h: 8.5–11.0 mmol/L. Routine screening was performed between 26 and 28 weeks. Patients with one or more risk factors for GDM (previous GDM, maternal age 40 years old, first-degree relative with diabetes, PCOS, Asian, Indian, and Aboriginal or Torres Strait Islander) underwent an early OGTT between 14 and 20 weeks.
The primary outcomes were birth weight above the 90th centile for gestational age, cesarean delivery, clinical neonatal hypoglycemia, and premature delivery (before 37 weeks’ gestation). These were selected as most representative of the criteria upon which current diagnostic criteria for GDM are based. Secondary outcomes were birth weight <10th or >95th centile, shoulder dystocia, neonatal respiratory distress, neonatal jaundice requiring phototherapy treatment, SCN or NICU admission, and hypertensive disorders of pregnancy (gestational hypertension, preeclampsia, and eclampsia). Birth weight centiles for each baby were calculated using Australian birth weight charts and twin-specific birth weight charts (Li et al., Reference Li, Umstad, Hilder, Xu and Sullivan2015).
The protocol was approved by the institutional research and ethics committee. Consent was not required as the data were retrospective, anonymized, and already collected within the existing hospital audit system.
Statistical Analysis
Maternal and neonatal characteristics were reported using descriptive statistics. Mean and standard deviations were reported for continuous variables, and number and percentage were reported for categorical variables. The frequency and centiles for the OGTT values were calculated. Univariate analyses of two variables, GDM and twin pregnancies, were performed to examine outcomes known to be associated with diagnosis of GDM. Discrete variables were analyzed using Fisher’s exact test or Pearson’s chi-squared and continuous variables using Student’s t test. Multivariate logistic regression was then performed to examine whether twins or GDM was a stronger predictor of outcomes of relevance. Variables were selected based on the univariate analyses. The multivariate linear model used in this study allowed us to assess the impact of variables, such as smoking, maternal age, and BMI on outcomes of interest. P-values <.05 were considered statistically significant and are reported with odds ratios and 95% confidence intervals. The data were analyzed with SAS University Edition Version 3.7 (SAS Institute, North Carolina, USA).
Results
A total of 15,138 women gave birth over the 2-year period, of whom 13,527 were eligible for the study (see Figure 1); 1611 women were excluded due to pregestational diabetes, congenital fetal anomalies, gestational age <22 weeks at delivery, or stillbirth. In our final cohort, 13,294 had singleton births and 233 had twins (annual incidence: 8.57 per 1000 births). The characteristics of the study sample are presented in Table 1. The mean age was 31.29 years, mean BMI was 24.74, and 1.63% women self-reported as Aboriginal or Torres Strait Islander. Of the 13,527 women, 1418 developed gestational diabetes (prevalence: 10.48%), and 39 of these women also had a twin pregnancy (see Table 2).
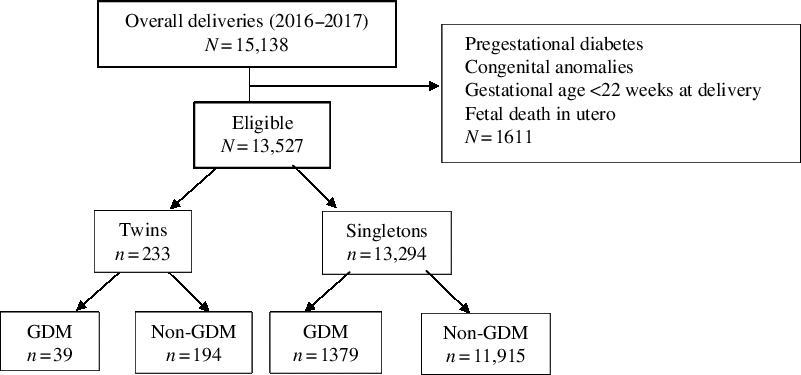
Fig. 1. Selection of the study group.
Table 1. Characteristics of the study participants and their newborns and frequency of outcomes

Table 2. Characteristics of the twin cohort compared to GDM cohort and their newborns and frequency of outcomes
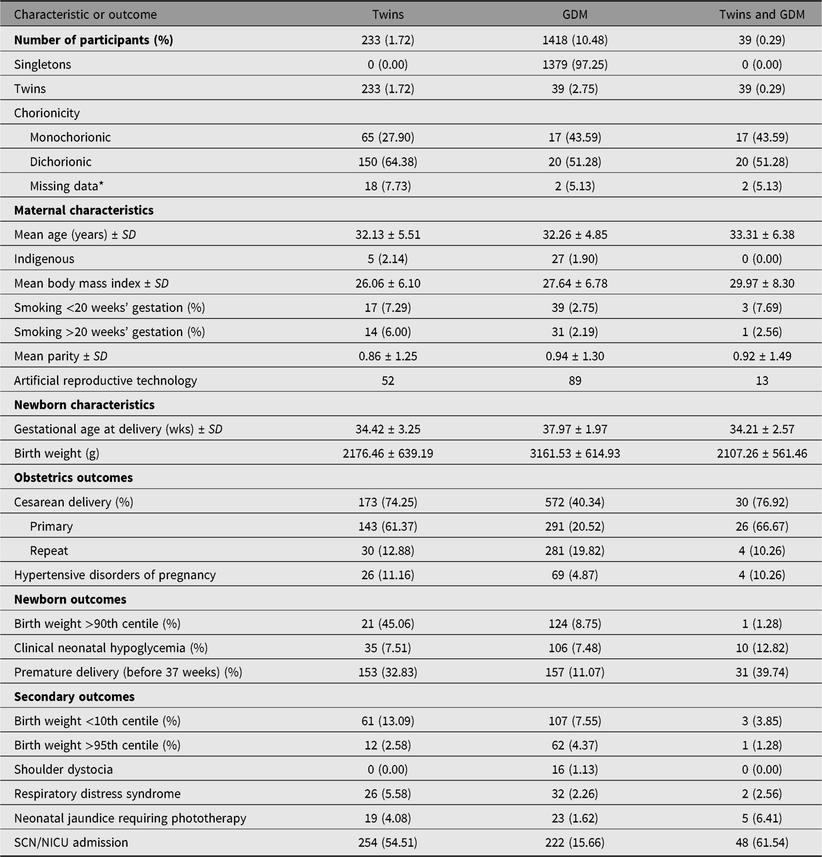
Note: GDM = gestational diabetes mellitus; SD = standard deviation; N/A = not applicable; SCN/NICU = Special Care Nursery or Neonatal Intensive Care Unit. *Missing data: chorionicity was not recorded in database.
Univariate analysis showed patients with GDM were more likely to have a cesarean delivery (OR 1.70, 95% CI [1.52, 1.91], p < .0001), preterm birth (OR 1.55, 95% CI [1.29, 1.84], p < .0001), or neonatal hypoglycemia (OR 5.05, 95% CI [3.93, 6.48], p < .0001) compared with those without GDM (see Table 3). Macrosomia (birth weight >90th or >95th centile), shoulder dystocia, and hypertensive disorder of pregnancy were not significantly increased with GDM. Twin pregnancies were significantly associated with all adverse outcomes compared to singletons except macrosomia (birth weight >90th and 95th centile; see Table 4). There were no cases of shoulder dystocia in the twin cohort.
Table 3. Perinatal outcomes associated with GDM: results from univariate logistic regression analysis
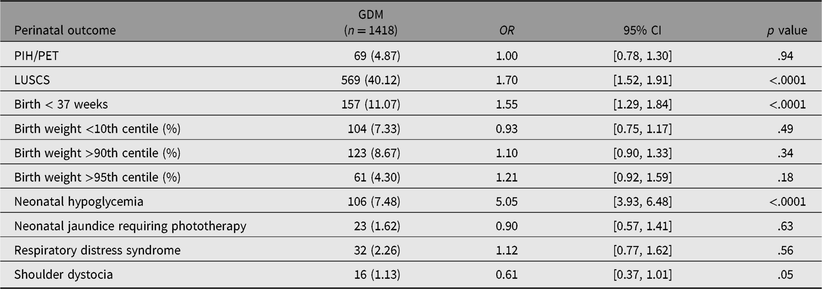
Note: OR = odds ratio; CI = confidence interval; GDM = gestational diabetes mellitus; PIH/PET = pregnancy-induced hypertension/preeclampsia; LUSCS = lower uterine segment cesarean section.
Table 4. Perinatal outcomes associated with twin pregnancies: results from univariate logistic regression analysis
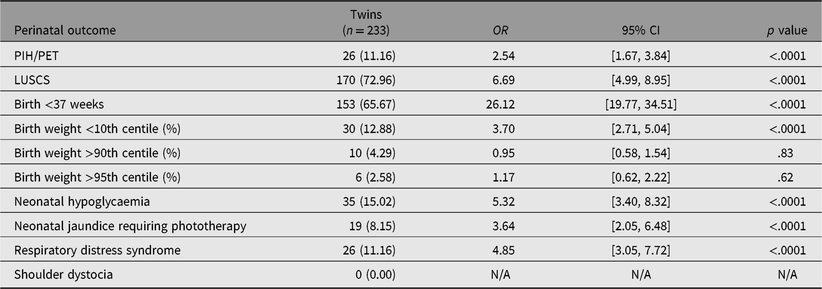
Note: OR = odds ratio; CI = confidence interval; GDM = gestational diabetes mellitus; PIH/PET = pregnancy induced hypertension/preeclampsia; LUSCS = lower uterine segment cesarean section.
Multivariate analyses were performed for cesarean delivery, preterm birth, and neonatal hypoglycemia (the outcomes significantly increased by a diagnosis of GDM). Twin pregnancies and GDM were both associated with increased risk of cesarean delivery, with twins showing a much stronger association. The strongest predictor for cesarean delivery was previous cesarean delivery (see Table 5). Twin pregnancy was significantly associated with preterm birth, but GDM was not (see Table 6). Twin pregnancy was no longer a statistically significant predictor for neonatal hypoglycemia but a diagnosis of GDM was. The strongest predictor for neonatal hypoglycemia was prematurity (see Table 7).
Table 5. Maternal demographics, obstetric and perinatal outcomes associated with caesarean delivery: results from multivariate logistic regression analysis
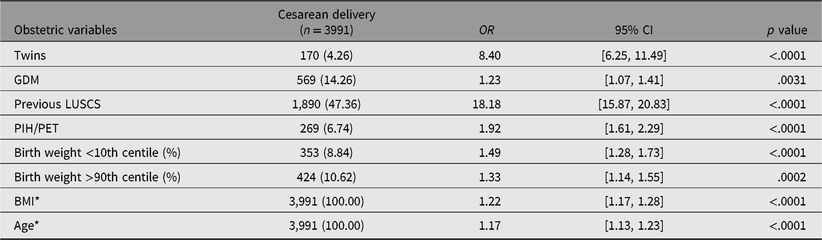
Note: OR = odds ratio; CI = confidence interval; GDM = gestational diabetes mellitus; LUSCS = lower uterine segment cesarean section; PIH/PET = pregnancy induced hypertension/preeclampsia; BMI = body mass index. *BMI and age: for every incremental increase of 5 units.
Table 6. Maternal demographics, obstetric and perinatal outcomes associated with preterm birth: results from multivariate logistic regression analysis

Note: OR = odds ratio; CI = confidence interval; PIH/PET = pregnancy induced hypertension/preeclampsia; BMI = body mass index; GDM = gestational diabetes mellitus. *BMI and age: for every incremental increase of 5 units.
Table 7. Maternal demographics, obstetric and perinatal outcomes associated with neonatal hypoglycemia: results from multivariate logistic regression analysis
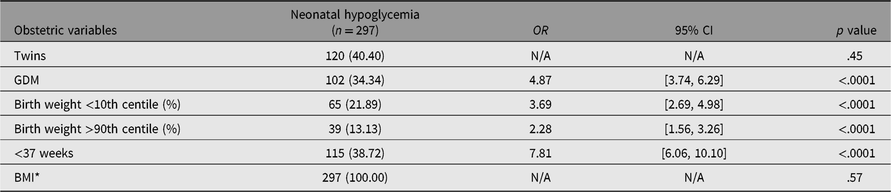
Note: OR = odds ratio; CI = confidence interval; PIH/PET = pregnancy induced hypertension/preeclampsia; BMI = body mass index; GDM = gestational diabetes mellitus. *BMI: for every incremental increase of 5 units.
A total of 237 women with twin pregnancies completed the OGTT. Seven were excluded due to incomplete results or did not meet inclusion criteria stated above. The mean fasting glucose level was 4.18 mmol/L (median 4.10 mmol/L, IQR 3.90–4.30 mmol/L); the mean 1-h fasting glucose level was 7.22 mmol/L (median 7.20, IQR 6.0–8.30 mmol/L), and 2-h plasma glucose level was 5.82 mmol/L (median 5.60, IQR 4.60–6.70 mmol/L).
Discussion
This study aimed to assess the impact of GDM and twin pregnancies, separately, on perinatal outcomes associated with a diagnosis of GDM and then to analyse whether GDM invokes an increased degree of risk on twin pregnancies. We have shown that a cohort of women with treated GDM will still have a higher risk of cesarean delivery, preterm birth <37 weeks, and neonatal hypoglycemia but, interestingly, not extremes of birth weight. Twin pregnancies, unsurprisingly, have a higher risk of all perinatal outcomes except macrosomia. When examined together, a twin pregnancy was a much higher predictor of cesarean delivery and preterm birth, but GDM was a higher predictor of neonatal hypoglycemia.
Univariate analysis showed that both twin pregnancy and GDM were significantly associated with cesarean delivery. Cesarean delivery is one of the known complications of GDM in singletons due to development of macrosomia (Okby et al., Reference Okby, Weintraub, Sergienko and Eyal2014). In twins, however, the risk of developing macrosomia and subsequent shoulder dystocia is low due to reduced growth potential in the third trimester (Sankilampi et al., Reference Sankilampi, Hannila, Saari, Gissler and Dunkel2013). Given that excessive fetal growth is less likely to occur in twin pregnancies, it seems improbable that GDM confers further risk of cesarean delivery in twin pregnancies. In our study, the OR for cesarean delivery in twins was much higher (OR 6.69, 95% CI [4.99, 8.95], p < .0001) compared to GDM (OR 1.7, 95% CI [1.52, 1.91], p < .0001). This was supported by multivariate analyses, which showed that twin pregnancy was more strongly associated with cesarean delivery compared with GDM (OR 8.40, 95% CI [6.25, 11.49] vs. OR 1.23, 95% CI [1.07, 1.41]). This is likely related to inherent indications for cesarean delivery associated with twin pregnancies, such as malpresentation, higher rates of placenta previa and fetal growth discrepancy, as well as patient and obstetrician preference. Furthermore, it was shown that obstetric history has a far greater impact compared to GDM on risk of cesarean delivery, with previous cesarean delivery, OR 18.18, 95% CI [15.87, 20.83], p < .0001 versus GDM, OR 1.23, 95% CI [1.07, 1.41], p = .0031.
Our results are supported by previous retrospective studies. When compared with twin pregnancies without GDM, no difference in cesarean delivery rates was seen in twins with GDM (Okby et al., Reference Okby, Weintraub, Sergienko and Eyal2014; Simoes et al., Reference Simoes, Queiros, Correia, Rocha, Dias and Blickstein2011). One study even reported significantly lower rates of cesarean delivery in twin pregnancies with glucose intolerance (Poulain et al., Reference Poulain, Duhamel, Garabedian, Cazaubiel, Rejou, Vambergue and Deruelle2015). However, the diagnostic criteria used in this study were not consistent with IADPSG, and thus, it is difficult to compare these results against our cohort. In contrast, a previous Australian study reported significantly higher rates of elective cesarean rates associated with GDM (Moses et al., Reference Moses, Webb, Lucas and Davis2003). Given the change in GDM diagnostic criteria since these results were published, again, it is difficult to compare these results to our newer cohort. Currently, there are limited prospective data on the impact of GDM on cesarean rates in twin pregnancies. The two landmark RCTs prompting a dramatic shift in GDM screening and management showed a reduction in cesarean delivery rates with treatment of GDM (Landon et al., Reference Landon, Spong, Thom, Carpenter, Ramin, Casey and Anderson2009) and an increased association with cesarean delivery with increased levels of maternal hyperglycemia (Metzger et al., Reference Metzger, Lowe, Dyer, Trimble, Chaovarindr, Coustan and Sacks2008). However, both trials excluded twin pregnancies. The only RCT to include twins showed no difference in cesarean delivery regardless of treatment and intervention in GDM (Crowther et al., Reference Crowther, Hiller, Moss, McPhee, Jeffries and Robinson2005); however, the numbers of twins were very small.
Our study found that twin pregnancies were associated with increased likelihood of preterm birth compared to GDM (OR 26.12, 95% CI [19.77, 34.51] vs. OR 1.55, 95% CI [1.29, 1.84]). This trend was supported in the multivariate analysis model in which twin pregnancies were the strongest predictor (OR 58.82, 95% CI [31.25, 125], p < .0001) while the relationship with GDM and PTB was nonsignificant. These results are consistent with a recent metaanalysis that found no difference in gestational age at delivery in GDM versus non-GDM twins (McGrath et al., Reference McGrath, Hocking, Scott, Seeho, Fulcher and Glastras2017). This metaanalysis included a number of large retrospective studies that have reported better birth outcomes associated with GDM in twin pregnancies. Foeller and colleagues found significantly lower PTB rates and higher birth weight compared to non-GDM twins (Foeller et al., Reference Foeller, Zhao, Szabo and Cruz2015). However, given the retrospective nature of the study, the degree of glycemic control or clinical management of GDM was not included, and thus, correlating energy supplies or demands to neonatal outcomes was not possible. Another recent study found no significant difference in gestational age at delivery between twin pregnancies with GDM and control (Fox et al., Reference Fox, Gerber, Saltzman, Gupta, Fishman, Klauser and Rebarber2016). Fox and colleagues (Reference Fox, Gerber, Saltzman, Gupta, Fishman, Klauser and Rebarber2016) suggested that improved glycemic control in twin pregnancies with GDM was associated with an increased risk of small for gestational age. However, the long-term impact of exposure to hyperglycemia in utero on epigenetics and metabolic complications in twin pregnancies are yet to be determined.
Preterm birth was the strongest predictor for neonatal hypoglycemia, almost double that of GDM (OR 7.81, 95% CI [6.06, 10.10] vs. OR 4.87, 95% CI [3.74, 6.29]). This is in line with previous literature regarding predictors of neonatal hypoglycemia with the strongest risk factor being early gestational age (Bromiker et al., Reference Bromiker, Perry, Kasirer, Einav, Klinger and Levy-Khademi2017; Kozen et al., Reference Kozen, Dassios, Kametas, Kapoor and Greenough2018). Notably, however, our multivariate analyses found that twin pregnancy was not significantly associated with this outcome. This contradicts our univariate analysis that found twin pregnancy was more likely to be complicated by neonatal hypoglycemia. The findings in our multivariate analyses challenge the classical concept of twin pregnancy being a risk factor for neonatal hypoglycemia (Bromiker et al., Reference Bromiker, Perry, Kasirer, Einav, Klinger and Levy-Khademi2017). We speculate the reason for this unexpected finding may be the fact that prematurity was a far stronger predictor in this model. Furthermore, there were only 35 recorded cases of neonatal hypoglycemia within our twin cohort. It may be that our sample of twins was not large enough to establish a strong enough relationship between twin pregnancy and neonatal hypoglycemia. Although not directly assessed in our study, the additive effects of GDM on twin pregnancies have previously been examined in retrospective studies. Nil significant difference was found in neonatal hypoglycemia rates in paired cohorts of well-controlled GDM and non-GDM twin pregnancies (Cho et al., Reference Cho, Shin, Yang, Ryu, Kim, Han and Choi2006; Guillen et al., Reference Guillen, Herranz, Barquiel, Hillman, Burgos and Pallardo2014). This suggests that the adverse outcomes associated with hyperglycemia in singleton pregnancies may not apply to twin pregnancies. Further prospective trials comparing the two cohorts are required to support these findings.
One of the aims of treating GDM in singleton pregnancies is to reduce the rate of macrosomia and its associated adverse birth and long-term outcomes. Interestingly, our study showed no significant association between GDM and birthweight >90th and 95th centiles. Previous evidence suggests that the treatment of GDM reduces the rate of macrosomia to similar levels to those without GDM (Jacqueminet & Jannot-Lamotte, Reference Jacqueminet and Jannot-Lamotte2010). It may be that because this study was performed in a tertiary center where GDM patients are managed in multidisciplinary clinics with close monitoring, the adverse effects of hyperglycemia may have been reduced in this cohort.
The current thresholds recommended by IADPSG for diagnosing GDM, including optimal timing for screening and target levels for glucose control, have not been tailored to twin pregnancies. Given the altered and exaggerated physiology in twin pregnancies, the universal adoption of one diagnostic criteria and management may not be appropriate (Yogev et al., Reference Yogev, Eisner, Hiersch, Hod, Wiznitzer and Melamed2014). We analyzed the frequency distribution of the OGTT results in our twin cohort and found that the 90th centile in this cohort correlated well with the HAPO defined values for GDM diagnostic criteria. Few studies have investigated the possibility of variations in GDM screening criteria in twin versus singleton pregnancies. Schwartz and colleagues highlighted that during the OGTT, the 3-h value was significantly elevated in twins but found no change in the overall OGTT result (Schwartz et al., Reference Schwartz, Daoud, Zazula, Goyert, Bronsteen, Wright and Copes1999). In more recent studies, a tendency for higher GCT and/or OGTT results in twin pregnancies was reported (Buhling et al., Reference Buhling, Henrich, Starr, Lubke, Bertram, Siebert and Dudenhausen2003; Yogev et al., Reference Yogev, Eisner, Hiersch, Hod, Wiznitzer and Melamed2014). Rebarber and colleagues (Reference Rebarber, Dolin, Fields, Saltzman, Klauser, Gupta and Fox2014) trialled different threshold scores for the GCT in twin pregnancies. They proposed that a higher 1-h GCT threshold maintained 100% sensitivity with a higher specificity and positive predictive value, thus resulting in fewer patients testing positive. Furthermore, Dinham et al. (Reference Dinham, Henry, Lowe, Nassar, Lui, Spear and Shand2016) found little benefit with increased diagnosis and treatment of GDM in twin pregnancies with the new IADPSG criteria. These results suggest that the physiological differences between twin pregnancies and singletons should be considered when assessing glucose tolerance in twin pregnancy. Larger studies are needed to define and tailor appropriate diagnostic thresholds and timing of GDM screening in twin pregnancies.
There were some limitations in our study. Given the retrospective design, we were unable to directly correlate perinatal outcomes with exposure to both twin pregnancies and GDM concomitantly. We were also unable to correlate glycemic control in GDM management with neonatal outcomes such as birth weight. Therefore, we cannot rule out the possibility that increased obstetric surveillance and tertiary-based management of GDM and twin pregnancies may have attenuated the differences in these cohorts versus controls. Due to the large sample size for most variables of interest, our study was able to detect significant differences in adverse perinatal outcomes related to twin pregnancies and GDM. However, the sample size for twin pregnancies may have been too small to identify trends in abnormal OGTT results in this cohort. Larger studies on glycemic trends in twin pregnancy and thus targeted diagnostic criteria of GDM are required. Furthermore, prospective studies assessing the management of GDM — specifically the effect of glycemic control — in twin pregnancies on perinatal outcomes are required.
Conclusion
In summary, our results indicate that twin pregnancy is an inherently high-risk condition as it was more strongly associated with all adverse perinatal outcomes compared to GDM except neonatal hypoglycemia. Evolving evidence suggests that the hyperglycemic environment in GDM may benefit the high energy and nutrient demands required in twin pregnancy. Strict GDM management could even potentiate rather than reduce adverse outcomes. We suggest that further prospective studies are required to evaluate the appropriate diagnostic criteria, and management for GDM in twins is required.
Acknowledgments
The authors thank Ms. Lynne Rigg at the Royal Women’s Quality and Safety Unit for assistance with data extraction.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of Interest
None.
Publication Ethics
The results from all patients’ assessments, pathology, and pregnancy outcomes were deidentified. Individual patient consent was waived as the study was approved by the Instiutional Ethics Committee (Royal Women’s Hospital Research and Ethics Committee) as a registered audit.












