Abstract
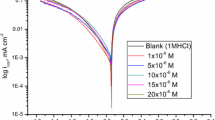
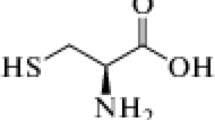
Corrosion kinetics of 99.6% aluminium covered by a thin spontaneously formed oxide film in hydrochloric acid solution with and without the presence of substituted N-aryl pyrroles was studied using electrochemical impedance spectroscopy and quasi steady-state polarization. Measurements were performed on a rotating disc electrode in an argon-deaerated solution in the temperature range 20 to 50°C. The addition of inhibitor considerably increases overvoltage of the cathodic process (HER) and shifts Ecorr to negative potential values. The activation energy of the hydrogen evolution reaction was Ea=50±5kJmol−1 and was not affected by the presence of inhibitor. The inhibitory action occurs by π-bonding between the adsorbed inhibitor molecules and the electrode surface. The electrode coverage follows the Langmuir adsorption isotherm with an adsorption equilibrium constant K=1.1–2.64×105dm3mol−1. The adsorption of organic compound prevents the adsorption of chloride ions and slow down the rate of corrosion.
Similar content being viewed by others
References
M. Pourbaix, `Atlas of Electrochemical Equilibrium Diagrams in Aqueous Solutions', NACE, Houston, Texas (1966), p. 499.
R. S. Alwitt, in `Oxides and Oxide Films', Vol.6 (edited by J.W. Diggle and A.K. Vijh), Marcel Dekker, New York (1976), p. 169.
F. D. Bogar and R. T. Foley, J. Electrochem. Soc. 119 (1972) 462.
G. Trabanelli, in `Corrosion Mechanisms' (edited by F. Mansfeld), Marcel Dekker, New York (1987), p. 119.
S. L. Granese, Corros. Sci. 44 (1988) 322.
S. L. Granese and B. M. Rosales, Proceedings of the 7th European Symposium on `Corrosion Inhibitors', Ann. Univ. Ferrara, N.S., Sez. V, Suppl. N.9 (1990), p. 73.
M. N. Desai, B. C. Thakar, P. M. Chhaya and M. H. Gandhi, Corros. Sci. 16 (1976) 9.
C. Monticelli, G. Brunoro and G. Trabanelli, Proceedings op. cit. ref. [6], p. 1125.
T. M. Salem, J. Horvath and P. S. Sidky, Corros. Sci. 18 (1978) 363.
D. Tromans, Corrosion 42 (1986) 601.
F. Tirbonod and C. Fiaud, Corros. Sci. 18 (1978) 139.
L. Garrigues, N. Pebere and F. Dabosi, Electrochim. Acta 41 (1996) 1209.
Z. Grubač, M. Metikoš-Huković, E. Stupnišek-Lisac and J. Vorkapić, Proceedings of Eurocorr 1991, Budapest (1991), p. 105.
E. Stupnišek-Lisac, M. Metikoš-Huković, D. Lenčić and J. Vorkapić-Furač, Corrosion 48 (1992) 924.
J. A. Maga, J. Agric. Food Chem. 29 (1981) 691.
J. R. Macdonald, `Impedance Spectroscopy', J. Wiley & Sons, New York (1987).
K. Juttner, Electrochim. Acta 35 (1990) 1501.
W. J. Lorentz and F. Mansfeld, Electrochim. Acta 31 (1986) 467.
F. Mansfeld, ibid. 35 (1990) 1533.
J. Vorkapić-Furač, M. Mintas, T. Burgenmeister and A. Mannschreck, J. Chem. Soc. Perkin Trans. 2 (1989) 713.
K. Imuro and T. Hanafusa, Bull. Chem. Soc. Jpn 49 (1976) 1364.
A. K. Vijh, J. Phys. Chem. 73 (1969) 506.
R. E. Mayer, J. Electrochem. Soc. 113 (1966) 1158.
B. G. Ateya, B. E. Anadouli and F. M. Nizamy, Corros. Sci. 24 (1984) 497, 509.
W. J. Lorentz and F. Mansfeld, ibid. 21 (1981) 647.
J. Bessone, C. Mayer, K. Juttner and W. J. Lorenz, Electrochim. Acta 28 (1983) 171.
H. J. Witt, C. Wijenberg and C. Crevecoeur, J. Electrochem. Soc. 126 (1979) 779.
C. M. Brett, Corros. Sci. 33 (1992) 203; idem, J. Appl. Electrochem. 20 (1990) 1000.
G. T. Burnstein and R. J. Cinderey, Corros. Sci. 33 (1992) 475.
G. T. Burnstein and R. J. Cinderey, ibid. 32 (1991) 1195.
H. J. W. Lenderink. M. V. D. Linden and J. H. Witt, ibid. 38 (1993) 1989.
J. B. Bessone, D. R. Salinas, C. Mayer, M. Ebert and W. J. Lorenz, Electrochim. Acta 37 (1992) 2283.
A. Frichet, P. Gimenez and M. Keddam, ibid. 38 (1993) 1957.
A. Despić and V. P. Parkhutik, in `Modern Aspects of Electrochemistry', Vol. 20 (edited by J. O'M. Bockris, R. E. White and B. E. Conway), Plenum Press, New York (1989), p. 401.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
METIKOŠ-HUKOVIĆ, M., BABIĆ, R. & GRUBAČ, Z. Corrosion protection of aluminium in acidic chloride solutions with nontoxic inhibitors. Journal of Applied Electrochemistry 28, 433–439 (1998). https://doi.org/10.1023/A:1003200808093
Issue Date:
DOI: https://doi.org/10.1023/A:1003200808093