Abstract
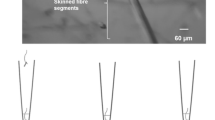
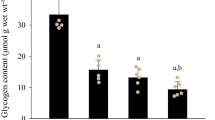
There is increasing evidence that endogenous glycogen depletion may affect excitation–contraction (E–C) coupling events in vertebrate skeletal muscle. One approach employed in physiological investigations of E–C coupling involves the use of mechanically skinned, single fibre preparations obtained from tissues stored under paraffin oil, at room temperature (RT: 20–24°C) and 4°C for several hours. In the present study, we examined the effect of these storage conditions on the glycogen content in three muscles frequently used in research on E–C coupling: rat extensor digitorum longus (EDL) and soleus (SOL) and toad iliofibularis (IF). Glycogen content was determined fluorometrically in homogenates prepared from whole muscles, stored under paraffin oil for up to 6 h at RT or 4°C. Control muscles and muscles stored for 0.5 and 6 h were also analysed for total phosphorylase (Phostotal) and phosphorylase a (Phos a) activities. No significant change was observed in the glycogen content of EDL and SOL muscles stored at RT for 0.5 h. In rat muscles stored at RT for longer than 0.5 h, the glycogen content decreased to 67.6% (EDL) and 78.7% (SOL) of controls after 3 h and 25.3% (EDL) and 37.4% (SOL) after 6 h. Rat muscles stored at 4°C retained 79.0% (EDL) and 92.5% (SOL) of glycogen after 3 h and 75.2% (EDL) and 61.1% (SOL) after 6 h. The glycogen content of IF muscles stored at RT or 4°C for 6 h was not significantly different from controls. Phostotal was unchanged in all muscles over the 6 h period, at both temperatures. Phos a was also unchanged in the toad IF muscles, but in rat muscles it decreased rapidly, particularly in EDL (4.1-fold after 0.5 h at RT). Taken together these results indicate that storage under paraffin oil for up to 6 h at RT or 4°C is accompanied by minimal glycogen loss in toad IF muscles and by a time- and temperature-dependent glycogen loss in EDL and SOL muscles of the rat.
Similar content being viewed by others
References
Armstrong RB and Phelps RO (1984) Muscle fibre type composition of the rat hindlimb. Am J Anat 171: 259–272.
Ballor DL and Poehlman ET (1993) Exercise intensity does not affect depression of resting metabolic rate during severe diet restriction in male Sprague-Dawley rats. J Nutr 123: 1270–1276.
Bergstrom J, Hermansen L, Hultman E and Saltin B (1967) Diet, muscle glycogen and physical performance. Acta Physiol Scand 71: 140–150.
Calder PC and Geddes R (1990) Post mortem glycogenolysis is a combination of phosphorolysis and hydrolysis. Int J Biochem 22: 847–856.
Chasiotis D (1985) Effects of adrenaline infusion on cAMP and glycogen phoshporylase in fast twitch and slow twitch rat muscles. Acta Physiol Scand 125: 537–540.
Chasiotis D, Edstrom L, Sahlin K and Sjoholm H (1985) Activation of glycogen phosphorylase by electrical stimulation of isolated fast twitch and slow twitch muscles from rat. Acta Physiol Scand 123: 43–47.
Cohen P (1983a) Phosphorylase kinase from rabbit skeletal muscle. Meth Enzymol 99: 243–250.
Cohen P (1983b) Protein phosphorylation and the control of glycogen metabolism in skeletal muscle. Philos Trans R Soc Lond B Biol Sci 302: 13–25.
Cohn C and Joseph D (1971) Feeding habits and daily rhythms in tissue glycogens in the rat. Proc Soc Exp Biol Med 137: 1305–1306.
Conlee RK, Rennie MJ and Winder WW (1976) Skeletal muscle glycogen content: diurnal variation and effects of fasting. Am J Physiol 231: 614–618.
Conlee RK, Mclane JA, Rennie MJ, Winder WW and Holloszy JO (1979) Reversal of phosphorylase activation in muscle despite continued contractile activity. Am J Physiol 237: R291-R296.
Conlee RK (1987) Muscle glycogen and exercise endurance: a twenty-year perspective. In: Pandolf KB (ed) Exercise and Sports Science Reviews. (vol. 15, pp. 1–28) MacMillan Publishing Co., New York.
Cuenda A, Nogues M, Henao F and Gutierrez-Merino C (1995) Interaction between glycogen phosphorylase and sarcoplasmic reticulum membranes and its functional role. J Biol Chem 270: 11998–12004.
DiMauro S, Trojaborg W, Gambetti P and Rowland LP (1971) Binding of enzymes of glycogen metabolism to glycogen in skeletal muscle. Arch Biochem Biophys 144: 413–422.
Entman ML, Keslensky SS, Chu A and Van Winkle WB (1980) The sarcoplasmic reticulum-glycogenolytic complex in mammalian fast twitch skeletal muscle. J Biol Chem 255: 6245–6252.
Ferreira LDM, Palmer TN and Fournier PA (1998) Prolonged exposure to halothane and associated changes in carbohydrate metabolism in rat muscles in vivo. J Appl Physiol 84: 1470–1474.
Fitts R (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94
Friden J, Seger J and Ekblom B (1989) Topographical localization of muscle glycogen: an ultrahistochemical study in the human vastus lateralis. Acta Physiol Scand 135: 381–391.
Green HJ (1991) How important is endogenous muscle glycogen to fatigue in prolonged exercise? Can J Physiol Pharmacol 69: 290–297.
Hoy JFY, Wah SY, Everett AW and Hughes S (1994) Plasticity of twitch muscle fibres of the toad, Bufo marinus, during adaptations to the seasons and to post metamorphic life. Basic Appl Myol 4: 369–380.
Hutber CA and Bonen A (1989) Glycogenesis in muscle and liver during exercise. J Appl Physiol 66: 2811–2817.
Hultman E (1967) Physiological role of muscle glycogen in man, with special reference to exercise. Circ Res 20–21 (Suppl.): I99-I114.
James DE and Kraegen EW (1984) The effect of exercise training on glycogen, glycogen synthase and phosphorylase in muscle and liver. Eur J Appl Physiol 52: 276–281.
Kushmerick MJ, Moerland TS and Wiseman RW (1992) Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP and Pi. Proc Natl Acad Sci USA 89: 7521–7525.
Lamb GD and Cellini MA (1999) High intracellular [Ca2+] alters sarcoplasmic reticulum function in skinned skeletal muscle fibres of the rat. J Physiol 519: 815–827.
Musch TI, Warfel BS, Moore RL and Larach DR (1989) Anaesthetic effects on liver and muscle glycogen concentrations: rest and post exercise. J Appl Physiol 66: 2895–2900.
Nguyen LT, Stephenson DG and Stephenson GMM (1998) Microfluorometric analyses of glycogen in freshly dissected, single skeletal muscles of the cane toad using a mechanically skinned fibre preparation. J Muscle Res Cell Motil 19: 631–638.
Nguyen LT and Stephenson GMM (1999) An electrophoretic study of myosin heavy chain expression in skeletal muscles of the toad Bufo marinus. J Muscle Res Cell Motil 20: 687–695.
Passonneau JV and Lowry OH (1993) Enzymatic Analysis: A Practical Guide (pp. 177–179). Humana Press, Totowa, NJ.
Pörtner HO, Branco LGS, Malvin GM and Wood SC (1994) A new function for lactate in the toad Bufo marinus. J Appl Physiol 76: 2405–2410.
Ren JM and Hultman E (1988) Phosphorylase activity in needle biopsy samples-factors influencing transformation. Acta Physiol Scand 133: 109–114.
Ren JM, Gulve EA, Cartee GD and Holloszy JO (1992) Hypoxia causes glycogenolysis without an increase in percent phosphorylase a in rat skeletal muscle. Am J Physiol 263: E1086-E1091.
Sahyun M (1931) On the carbohydrate of the muscles of the frog (Rana pipiens). J Biol Chem 94: 29–38.
Severinsen T and Munch IC (1999) Body core temperature during food restriction in rats. Acta Physiol Scand 165: 299–305.
Stephenson DG, Nguyen LT and Stephenson GMM (1999) Glycogen content and excitation-contraction coupling in mechanically skinned muscle fibres of the cane toad. J Physiol 519: 177–187.
Stephenson GM and Stephenson DG (1996) Effects of ammonium ions on the depolarization-induced and direct activation of the contractile apparatus in mechanically skinned fast-twitch skeletal muscle fibres of the rat. J Muscle Res Cell Motil 17: 611–616.
Villa Moruzzi E, Bergamini E and Gori Bergamini Z (1981) Glycogen metabolism and the function of fast and slow muscles of the rat. Pflugers Arch 391: 338–342.
Villa Moruzzi E and Bergamini E (1983) Effect of denervation on glycogen metabolism in fast and slow muscle of rat. Muscle Nerve 6: 356–366.
Wanson JC and Drochmans P (1972) Role of the sarcoplamsic reticulum in glycogen metabolism. J Cell Biol 54: 206–224.
Witzmann FA, Kim DH and Fitts RH (1983) Effect of hindlimb immobilization on the fatigability of skeletal muscle. J Appl Physiol 54: 1242–1248.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goodman, C.A., Stephenson, G.M.M. Glycogen stability and glycogen phosphorylase activities in isolated skeletal muscles from rat and toad. J Muscle Res Cell Motil 21, 655–662 (2000). https://doi.org/10.1023/A:1005628500892
Issue Date:
DOI: https://doi.org/10.1023/A:1005628500892