Abstract
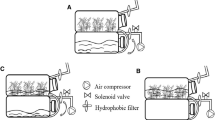
Protocorm-like bodies (PLBs) formed on leaf segmentsin vitro were used as explants for bioreactor cultures. Continuous immersion cultures (air lift column and air lift-balloon bioreactor), and temporary immersion cultures (with or without charcoal filter attached) were used for the culture of PLB sections. A temporary immersion culture with charcoal filter attached was most suitable for PLB culture. About 18,000 PLBs were harvested from 20 g of inoculum (∼1000 PLB sections) in 2 l Hyponex medium after 8 weeks of incubation. Aeration in a bioreactor at 0.5 or 2.0 volume of air per volume of medium min−1 (vvm) yielded similar levels of biomass production. PLBs grown in bioreactors were cultured on solid Murashige and Skoog, Vacin and Went, Knudson C, Lindemann and Hyponex media. Hyponex medium was found to be suitable for conversion of PLBs into plantlets and 83% of PLBs transformed into plantlets on this medium. The feasibility of using PLBs for large-scale micropropagation was evaluated for scaled-up liquid cultures in bioreactors, rate of proliferation, and regeneration.
Similar content being viewed by others
References
Aitken-Christie J, Kozai T & Takayama S (1995) Automation in plant tissue culture. General introduction and overview. In: Aitken-Christe J, Kozai T & Smith MAL (eds) Automation and Environmental Control in Plant Tissue Culture (pp 1–18). Kluwer Academic Publishers, Dordrecht
Akita M & Takayma S (1988) Mass propagation of potato tubers using jar fermentor techniques. Acta Hortic. 230: 55–61
Arditti J & Ernst R (1993) Micropropagation of Orchids. John Wiley & Son, Inc., New York
Cantliffe DJ, Bieniek ME & Harrell RC (1993) A system approach to developing and automated synthetic seed model. In: Soh WY, Liu JR & Komamine A (eds) Advances in the Developmental Biology and Biotechnology of Higher Plants (pp 160–196). The Korean Society of Plant Tissue Culture, Korea
Griesbach RJ (1983) The use of indoleactylamino acids in the in vitro propagation of Phalaenopsis orchids. Sci. Hortic. 19: 363–366
Gupta PK, Timmis R & Carlson WC (1993) Somatic embryogenesis: A possible tool for large-scale propagation of forestry species. In: Soh WY, Liu JR & Komamine A (eds) Advances in Developmental Biology and Biotechnology of Higher Plants (pp 18–37). The Korean Society of Tissue Culture, Korea
Ilan A, Ziv m & Halevy AH (1995) Propagation and corm development of Brodiaea in liquid cultures. Sci. Hortic. 63: 101–112
Kano K (1965) Studies on the media for orchid seed germination. Mem. Fac. Agric. Kagawa Univ. 20: 1–68
Knudson L (1946) A new nutrient solution for germination of orchid seed. Amer. Orchid Soc. Bull. 15: 214–217
Leathers RR, Smith MAL & Aitken-Christie J (1995) Automation of the bioreactor process for mass propagation and secondary metabolites. In: Aitken-Christe J, Kozai T & Smith MAL (eds) Automation and Environmental Control in Plant Tissue Culture (pp 187–214). Kluwer Academic Publishers, Dordrecht
Lilien-Kipnis H, Ziv M & Kahany S (1990) Proliferation of plantlet regeneration from inflorescence derived Nerine explants cultured in vitro. In: De Jong (ed) Integration of in vitro Techniques in Ornamental Plant Breeding (pp 28–33). CPO Center for Plant Breeding Research, Wageningen, The Netherlands
Lin CC (1986) In vitro culture of flower stalk internodes of Phalaenopsis and Doritaenopsis. Lindleyana 1: 158–163
Lindemann EGP, Gunckel JE & Davidson OW (1970) Meristem cutlure of Cattleya. Amer. Orchid Soc. Bull. 39: 100–127
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Preil W (1991) Application of bioreactors in plant propagation. In: Debergh PC & Zimmerman RH (eds) Micropropagation-Technology and Application (pp 425–445). Kluwer Academic Publishers, Dordrecht
Preil W & Beck A (1991) Somatic embryogenesis in bioreactor culture. Acta Hortic. 289: 179–192
Sigeoka T, Kozumi Y & Kawamura M (1994) Mass propagation of shoots of Stevia rebuniana using large scale bioreactor. Plant Cell Rep. 13: 180–183
Seon JH, Kim YS, Son SH & Paek KY (2000) The Fed-batch culture system using bioreactor for the bulblet production of oriental lillies. Acta Hortic. 520: 53–59
Son SH, Choi SM, Yun SR, Kwon UW, Lee YH & Paek KY (1999) Large scale culture of plant cell and tissue by bioreactor system. J. Plant Biotech. 1: 1–7
Stuart DA, Strickland SG & Walkar KA (1987) Bioreactor production of alfalfa somatic embryos. HortScience 22: 800–809
Takahashi S, Matsubara K, Yamagata H & Morimoto T (1992) Micropropagation of virus free bulblets of Lilium longiflorum by tank culture 1. Development, culture method and large scale propagation. Acta Hortic. 319: 83–88
Vacin E & Went FW (1949) Some pH changes in nutrient solutions. Bot Gaz. 110: 605–613
Vanderschaege AM & Debergh PC (1988) Automation of tissue culture manipulations in the final stages. Acta Hortic. 227: 399–401
Vasil IK (1994) Automation of plant propagation. Plant Cell Tiss. Org. Cult. 39: 105–108
Watad AA, Alper Y, Stav R & Levin R (1999) Mechanization of micropropagation In: Altmann A, Ziv M & Izhar G (eds) Plant Biotechnology and In Vitro Biology in the 21st century (pp. 663–666). Kluwer Academic Publishers, Dordrecht
Ziv M (1989) Enhanced shoot and cormlet proliferation in liquid cultured gladiolus buds by growth retardants. Plant Cell Tiss. Org. Cult. 17: 101–110
Ziv M (1992a) The use of growth retardants for the regeneration and acclimatization of in vitro plants. In: Karssen CM, Von Loon LC & Vreugdenhil (eds) Progress in Plant Growth Regulation (pp 809–817). Kluwer Academic Publishers, Dordrecht
Ziv M (1992b) Morphogenic control of plants micropropagated in bioreactor cultures and its possible impact on acclimatization. Acta Hortic. 319: 119–124
Ziv M (1999) Organogenic plant regeneration in bioreactors. In: Altmann A, Ziv M & Izhar G (eds) Plant Biotechnology and In Vitro Biology in the 21st Century (pp 673–676). Kluwer Academic Publishers, Dordrecht
Ziv M & Hadar A (1991) Morphogenic pattern of Nephrolepis exalata cv. Bostoniensis in agar or liquid cultures: implications for micropropagation. Isr. J. Bot. 40: 7–16
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
So Young, P., Murthy, H. & Kee Yoeup, P. Mass multiplication of protocorm-like bodies using bioreactor system and subsequent plant regeneration in Phalaenopsis . Plant Cell, Tissue and Organ Culture 63, 67–72 (2000). https://doi.org/10.1023/A:1006420116883
Issue Date:
DOI: https://doi.org/10.1023/A:1006420116883