Abstract
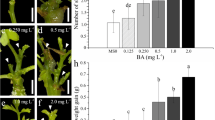
Internode explants collected from in vitro grown shoots of two clones of Fagus sylvatica L. (European beech) and five clones of F. orientalis Lipski (Oriental beech) were used to evaluate their bud regeneration capacity. Adventitious shoot-buds formed on callus, which developed from internode segments cultured in a Woody Plant Medium supplemented with different concentrations of either thidiazuron (TDZ) or benzyladenine (BA). After 4 weeks of culture on induction media, the explants were transferred to a proliferation medium supplemented with 2.2 μM BA, 9.1 μM zeatin and 2.9 μM indole-3-acetic acid (IAA) for another 8 weeks. Medium containing TDZ was much more efficient than medium containing BA in inducing adventitious buds, the optimal TDZ concentration being 4.5 μM and the optimal BA concentration 17.8 μM. Genotypic variation in shoot regeneration capacity was observed among the two Fagus species and between clones within each species, with a significant interaction between TDZ concentration and genotype regarding mean bud number. Thidiazuron induction medium supplemented with a range of individual auxins was investigated, and it was found that IAA or indole-3-butyric acid at 2.9 μM enhanced the bud forming capacity of explants. Morphogenic response varied significantly with the position of the internode along the stem. The highest regeneration potential was obtained from apical internodes, while those distal to the apex were the least productive. Elongated shoots of adventitious origin can be readily proliferated by axillary branching.
Similar content being viewed by others
References
Baker BS & Bhatia SK (1993) Factors affecting adventitious shoot regeneration from leaf explants of quince (Cydonia oblonga). Plant Cell Tiss. Org. Cult. 35: 273–277
Barker MJ, Pijut PM, Ostry ME & Houston DR (1997) Micropropagation of juvenile and mature American beech. Plant Cell Tiss. Org. Cult. 51: 209–213
Bergmann BA & Moon H-K (1997) In vitro adventitious shoot production in Paulownia. Plant Cell Rep. 16: 315–319
Bergmann BA & Stomp AM (1994) Effect of genotype on in vitro adventitious shoot formation in Pinus radiata and correlations between pairs of phenotypic traits during in vitro shoot development. Plant Cell Tiss. Org. Cult. 39: 185–194
Bretagne B, Chupeau M-C, Chupeau Y & Fouilloux G (1994) Improved flax regeneration from hypocotyls using thidiazuron as a cytokinin source. Plant Cell Rep. 14: 120–124
Brown CW & Thorpe TA (1986) Plant regeneration by organogenesis. In: Vasil IK (ed) Cell Culture and Somatic Cell Genetics of Plants, Vol 3 (pp 49–65). Academic Press, Inc. Orlando
Burger D & Hackett W (1986) Gradients of adventitious bud formation on excised epicotyl and root sections of Citrus. Plant Sci. 43: 229–232
Chalupa V (1996) Fagus sylvatica L. (European beech). In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry, Vol 35, Trees IV (pp 138–154). Springer-Verlag, Berlin Heidelberg
Coleman GD & Ernst SG (1989) In vitro shoot regeneration of Populus deltoides: effect of cytokinin and genotype. Plant Cell Rep. 8: 459–462
Cuenca B & Vieitez AM (1999) Histological study of in vitro development of adventitious buds on leaf explants of Oriental beech (Fagus orientalis Lipski). In Vitro Cell. Dev. Biol. Plant 35: 326–332
Eapen S, Tivarekar S & George L (1998) Thidiazuron-induced shoot regeneration in pigeonpea (Cajanus cajan L.). Plant Cell Tiss. Org. Cult. 53: 217–220
Graham J, Iasi L & Millan S (1997) Genotype-specific regeneration from a number of Rubus cultivars. Plant Cell Tiss. Org. Cult. 48: 167–173
Huetteman CA & Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss. Org. Cult. 33: 105–119
Kim MK, Sommer HE, Bongarten BC & Merkle SA (1997) High-frequency induction of adventitious shoots from hypocotyl segments of Liquidambar styraciflua L. by thidiazuron. Plant Cell Rep. 16: 536–540
Lloyd G & McCown BH (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb. Proc. Int. Plant Propagators’ Soc. 30: 421–427
Lu C-Y (1993) The use of thidiazuron in tissue culture. In Vitro Cell. Dev. Biol. 29P: 92–96
Meier K & Reuther G (1994) Factors controlling micropropagation of mature Fagus sylvatica. Plant Cell Tiss. Org. Cult. 39: 231–238
Mencucini M & Rugini E (1993) In vitro shoot regeneration from olive cultivar tissues. Plant Cell Tiss. Org. Cult. 32: 283–288
Murch SJ, Krishnaraj S & Saxena PK (1997) TDZ-induced morphogenesis of Regal Geranium (Pelargonium domesticum): a potential stress response. Physiol. Plant. 101: 183–191
Murthy BNS, Murch SJ & Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell. Dev. Biol. Plant 34: 267–275
Srivastava S, Glock H & Zhang Z (1991) Tissue culture studies on chinese poplar (Populus tomentosa). Silv. Genet. 40: 247–248
Tzfira T, Jensen CS, Vainstein A & Altman A (1997) Transformation and regeneration of transgenic aspen plants via shoot formation from stem explants. Physiol. Plant. 99: 554–561
Vieitez AM, Ferro EM & Ballester A (1993) Micropropagation of Fagus sylvatica L. In Vitro Cell. Dev. Biol. 29P: 183–188
Vieitez AM & San-José MC (1996) Adventitious shoot regeneration from Fagus sylvatica leaf explants in vitro. In Vitro Cell. Dev. Biol. Plant 32: 140–147
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cuenca, B., Ballester, A. & Vieitez, A. In vitro adventitious bud regeneration from internode segments of beech. Plant Cell, Tissue and Organ Culture 60, 213–220 (2000). https://doi.org/10.1023/A:1006428717309
Issue Date:
DOI: https://doi.org/10.1023/A:1006428717309