Abstract
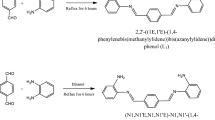
Three novel tridentate Schiff base ligands derived from␣the 3-hydroxysalicylaldehyde (H2L1), 4-hydroxysalicylaldehyde (H2L2) and 5-bromosalicylaldehyde (H2L3) with a new amine N-(pyridyl)-2-hydroxy-3-methoxy-5-aminobenzylamine (2) have been prepared. The ligands and their metal complexes have been characterized by elemental analyses, conductivity and magnetic susceptibility measurements, i.r., electronic absorption and 1H and 13C n.m.r. spectroscopy. All complexes are binuclear and, in some, the H2O molecules are coordinated to the metal ion. Antimicrobial activities of the ligands and their complexes have been tested against to the Bacillus subtilis IMG 22 (bacteria), Micrococcus luteus LA 2971 (bacteria) Saccharamyces cerevisiae WET 136 (yeast), and Candida albicans CCM 314 (yeast). Thermal properties of all complexes have been studied by t.g. and d.t.a techniques.
Similar content being viewed by others
References
G. Albertin, E. Bordignon and A. A. Orio, Inorg. Chem., 14, 1411 (1975).
K. D. Karlin and J. Zubieta (Eds), Copper Coordination Chemistry: Biochemical and Inorganic Perspectives, Adenine Press, New York, 1983, p. 43.
R. H. Holm, G. W. Everett and A. Chakravorty, Prog. Inorg. chem., 7, 83 (1966).
K. Kawakami, M. Miya-Uchi and T. Tanaka, J. Inorg. Nucl. Chem., 33, 3773 (1971).
J. I. Bullock and H. A. Tajmir-Riahi, J. Chem. Soc., Dalton Trans., 36 (1978).
P. Bam®eld, J. Chem. Soc. A, 804 (1967).
M. R. Mahmoud and M.T. El-Haty, J. Inorg. Nucl. Chem., 42, 349 (1980).
C. N. Reilley, R. W. Schimid and F. A. Sadek, J. Chem. Educ., 36, 555 (1959).
C. H. Collins, P. M. Lyne and J. M. Grange Microbiological Methods3th edn., Butterworth, 1989, p. 10.
W. J. Geary, Coord. Chem. Rev., 7, 81 (1971).
M. Tümer, H. Köksal and S. Serin, Synth. React. Met.-Org. Chem., 26, 1589 (1996).
H. Köksal, M. Tümer and S. Serin, Synth. React. Met.-Org. Chem., 261577 (1996).
M. Goldstein, E. Mooney. A. Anderson and H. A. Gebbie, Spectrochim Acta 21, 105 (1965).
L. Rohrschneider, Anal. Chem., 45, 1241 (1973).
M. M. Mostafa, A. El-Hammid, M. Shallaby and A. A. El-Asmy, Transition Met. Chem., 6, 303 (1981).
M. J. M. Cambell, Coord. Chem. Rev., 15, 279 (1975).
K. N. Raymond and F. Basolo, Inorg. Chem., 5, 1632 (1966).
M. R. Truter, Chem. Brit., 7, 203 (1971).
M. Tümer, H. Köksal, S. Serin and S. Patat, Synth. React. Met.-Org. Chem., 27, 59 (1997).
M. Tümer, B. Erdo_gan, H. Köksal, S. Serin and M. Y. Nutku, Synth. React. Met.-Org. Chem., 28, 529 (1998).
L. Lindoy, W. E. Moody and D. Taylor, Inorg. chem., 16, 1962 (1977).
W. Radecka-Paryzek and E. Jankowska, Inorg. Chim. Acta, 134, 179 (1987).
W. Bryzska and A. Krol, Thermochim. Acta, 223, 241 (1993).
M. A. Donia, A. S. Amer, M. R. Issa and M. Gaber, Trans. Met. Chem., 15, 465 (1990).
P. G. Lawrance, P. L. Harold and O. G. Francis, Antibiotic and Chemotheraphy, 5, 1597 (1980).
S. Rich and J. G. Horsfall, Phytopathology, 42, 457 (1952).
Rights and permissions
About this article
Cite this article
Tümer, M., Köksal, H., Sener, M.K. et al. Antimicrobial activity studies of the binuclear metal complexes derived from tridentate Schiff base ligands. Transition Metal Chemistry 24, 414–420 (1999). https://doi.org/10.1023/A:1006973823926
Issue Date:
DOI: https://doi.org/10.1023/A:1006973823926