Abstract
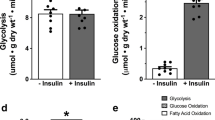
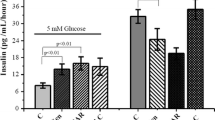
It is now widely accepted that insulin stimulation of glucose uptake by muscle cells is due to the activation of protein kinase B, leading to the recruitment of glucose transporter proteins from an intracellular compartment to the plasma membrane. Vanadate is a protein tyrosine phosphatase (PTP) inhibitor and a known insulin mimetic agent. Vanadate causes an increase of glucose transport in various tissues, but the mechanism of stimulation is not clearly understood. Hence in the present study, we have compared the mechanism of 2-deoxy-D-glucose transport induced by vanadate and insulin in isolated rat cardiomyocytes. Vanadate stimulated deoxyglucose transport in a time- and concentration-dependent manner. Insulin (100 nM) and vanadate (5 mM) stimulated 2-deoxy-D-glucose transport on an average by 3- and 2-fold respectively over basal values. The stimulation of glucose transport was accompanied by an activation of protein kinase B (PKB). This study also revealed that the activation of PKB and stimulation of 2-deoxyglucose uptake by vanadate and insulin are inhibited by treatment with wortmannin, a specific inhibitor of phoshatidylinositol 3-kinase (PI 3-kinase). Hence, we conclude that both insulin and vanadate follow the same signalling pathway downstream of PI 3-kinase to stimulate 2-deoxy-D-glucose transport.
Similar content being viewed by others
References
James DE, Kraegen EW, Chisholm DJ. Muscle glucose metabolism in exercising hearts: comparison with insulin stimulation. Am J Physiol 1985;248:E575-E580.
Kainulainen H, Breiner M, Schurmann A, Marttinen A, Virjo A, Joost HG. In vivo glucose uptake and glucose transporter proteins GLUT1 and GLUT4 in heart and various Types of skeletal muscle from streptozotocin-diabetic rats. Biochim Biophys Acta 1994;1225:275-282.
Kolter T, Uphues I, Eckel J. Molecular analysis of insulin resistance in isolated ventricular cardiomyocytes of obese Zucker rats. Am J Physiol 1997;273:E59-E67.
Fischer Y, Thomas J, Rosen P, Kammermeier H. Action of metformin on glucose transport and glucose transporter GLUT1 and GLUT4 in heart muscle cells from healthy and diabetic rats. Endocrinology 1995;136:412-420.
Liu LS, Tanaka H, Ishii S, Eckel J. The new antidiabetic drug MCC-555 acutely sensitizes insulin signalling in isolated cardiomyocytes. Endocrinology 1998;139:4531-4539.
Lochner A, Pentz A, Williams K, Tromp E, Harper IS. Substrate effects on sarcolemmal permeability in the normoxic and hypoxic perfused rat heart. Basic Res Cardiol 1996;91:64-78.
Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction: effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57-65.
Rodrigues B, Cam MC, McNeill JH. Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem 1998;180:53-57.
Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 1998;40:239-247.
Egert S, Nguyen N, Brosius FC, Schwaiger M. Effects of wortmannin on insulin-and ischemia-induced stimulation of GLUT4 translocation and FDG uptake in perfused rat hearts. Cardiovasc Res 1997;35:283-293.
Sheperd PR, Nave BT, Rincon J, et al. Involvement of phosphoinositide 3-kinase in insulin stimulation of MAP-kinase and phosphorylation of protein kinase B in human skeletal muscle: Implications for glucose metabolism. Diabetologia 1997;40:1172-1177.
Wang Q, Bilan PJ, Tsakiridis T, Hinek A, Klip A. Actin filaments participate in the relocalization of phosphatidylinositol 3-kinase to glucose transport containing compartments and in the stimulation of glucose uptake in 3T3-L1 adipocytes. Biochem 1998;331:917-928.
Lefebvre V, Mechin MC, Louchn MP, Rider MH, Hue L. Signalling pathways involved in the activation of heart 6-phosphofructo-2-kinase by insulin. J Biol Chem 1996;271:22289-22292.
Fischer Y, Kamp J, Thomas J, et al. Signal mediating stimulation of cardiomyocyte glucose transport by the α-adrenergic agonist phenylephrine. Am J Physiol 1996;270:C1211-C1220.
Kohn AD, Kovacina K, Roth RA, Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J 1995;14:4288-4295.
Tanti JF, Grillo S, Gremeaux T, Coffer PJ, Obberghen EV, Marchand-Brustel YL. Potential role of portein kinase B in glucose transporter 4 translocation in adipocytes. Endocinology 1997;138:2005-2010.
Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kimase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem 1996;271:31372-31378.
Ueki K, Yamamoto-Honda R, Kaburagi Y, et al. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem 1998;273:5315-5322.
Hajduch E, Alessi DR, Hemmings BA, Hundal HS. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and activation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 1998;47:1006-1013.
Cong LN, Chen H, Li Y, et al. Physiological role of Akt in insulin-stimulated translocation ofGLUT4in transfected rat adipose cells. Mol Endocrinol 1997;11:1881-1890.
Wang Q, Somwar R, Bilan PJ, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol 1999;19:4008-4018.
Badmev V, Prakash S, Maheed M. Vanadium: a review of its potential role in the fight against diabetes. J Alter Compli Med 1999;8:273-291.
Morinville A, Maysinger D, Shaver A. From vanadis to atropos: vanadium compounds as pharmacological tools in cell death signalling. Trends Pharm Sci 1998;19:452-460.
Barceloux DG. Vanadium. Clin Toxicol 1999;37:265-278.
Poucheret P, Verma S, Grynpas MD, McNeill JH. Vanadium and diabetes. Mol Cell Biochem 1998;188:73-80.
Fantus IG, Tsiani E. Multifunctional actions of vanadium compounds on insulin signalling pathways: evidence for preferential enhancement of metabolic versus mitogenic effects. Mol Cell Biochem 1998;182:109-119.
Kopp SJ, Daar J, Paulson DJ, Ramano FD, Laddaga R. Effects of oral vanadyl treatment on diabetes-induced alterations in the heart GLUT-4 transporter. J Mol Cell Cardiol 1997;29:2355-2362.
Takeuchi K, McGowan FX, Glynn P, et al. Glucose transporter upregulation improves tolerance in hypertrophied failing heart. Circulation 1998;98:II234-II241.
Tamura S, Brown TA, Whipple JH, et al. Anovel mechanism for the insulin-like effect of vanadate on glycogen synthase in rat adipocytes. J Biol Chem 1984;259:6650-6658.
Shechter Y. Insulin-mimetic effects of vanadate: possible implications for future treatment of diabetes. Diabetes 1990;39:1-5.
Strout HV, Vicario PP, Saperstein R, Slater EE. The insulin mimetic effect of vanadate is not correlated with insulin receptor tyrosine kinase activity nor phosphorylation in mouse diaphragm in vivo. Endocrinology 1989;124:1918-1924.
Venkatesan N, Avidan A, Davidson MB. Antidiabetic action of vanadyl in rats independent of in vivo insulin receptor kinase activity. Diabetes 1991;40:492-498.
Berger J, Hayes N, Szalkowski DM, Zhang B. PI 3-kinase activation is required for insulin stimulation of glucose transport into L6 myotubes. Biochem Biophys Res Commun 1994;205:570-576.
Tsiani E, Bogdanovic E, Sorisky A, Nagy L, Fantus IG. Tyrosine phosphatase inhibitors, vanadate and pervanadate, stimulate glucose transport andGLUTtranslocation in muscle cells by a mechanism independent of phosphatidylinositol 3-kinase and protein kinase C. Diabetes 1998;47:1676-1686.
Cortright RN, Azevedo Jr JL, Hickey MS, Tapscott EB, Dohm GL. Vanadate stimulation of 2-deoxyglucose transport is not mediated by PI 3-kinase in human skeletal muscle. Biochim Biophys Acta 1997;1358:300-306.
Molero JC, Martinez C, Adres A, Satrustegui J, Carrascosa JM. Vanadate fully stimulates insulin receptor substrate-1 associated phosphatidylinositol 3-kinase activity in adipocytes from young and old rats. FEBS Lett 1998;425:298-304.
Fischer Y, Rose H, Kammermeir H. Highly insulinresponsive isolated rat heart muscle cells yielded by a modified isolation method. Life Sci 1991;49:1679-1688.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265-275.
Bradford MM. Arapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;71:248-254.
Tsiani E, Abdullah N, Fantus IG. Insulin-mimetic agents vanadate and pervanadate stimulate glucose but inhibit amino acid uptake. Am J Physiol 1997;272:C156-C162.
Shisheva A, Shechter Y. Mechanism of pervanadate stimulation and potentiation of insulin-activated glucose transport in rat adipocytes: dissociation from vanadate effect. Endocrinology 1993;133:1562-1568.
Ida M, Imai K, Hashimoto S, Kawashima H. Pervanadate stimulation of wortmannin-sensitive and-resistant 2-deoxyglucose transport in adipocytes. Biochem Pharmacol 1996;51:1061-1067.
Morioka M, Fukunaga K, Kawano T, et al. Serine/threonine phosphatase activity of calcineurin is inhibited by sodium orthovanadate and dithiothreitol reverses the inhibitory effect. Biochem Biophys Res Commun 1998;253:342-345.
Swarup G, Cohen S, Garbers D. Inhibition of membrane phosho tyrosyl protein phosphatase activity by vanadate. Biochem Biophys Res Commun 1982;107:1104-1109.
Pandey SK, Anand-Srivastava MB, Srivastava AK. Vanadyl sulfate-stimulated glycogen synthesis is associated with activation of phosphatidylinositol 3-kinase and is independent of insulin receptor tyrosine phosphorylation. Biochemistry 1998;37:7006-7014.
Elberg G, He Z, Li J, Sekar N, Shechter Y. Vanadate activates membranous nonreceptor protein tyrosine kinase in rat adipocytes. Diabetes 1997;46:1684-1690.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Donthi, R.V., Huisamen, B. & Lochner, A. Effect of Vanadate and Insulin on Glucose Transport in Isolated Adult Rat Cardiomyocytes . Cardiovasc Drugs Ther 14, 463–470 (2000). https://doi.org/10.1023/A:1007876703644
Issue Date:
DOI: https://doi.org/10.1023/A:1007876703644