Abstract
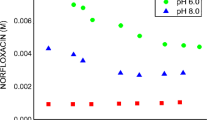
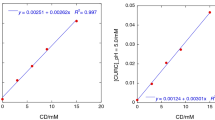
The effect of the type of cyclodextrin (α-, β-, γ-,hydroxypropyl-β-CD) and of hydroxyacid (tartaric, citric, gluconic,malic, lactic) on the solubility enhancement by multicomponent complexationof econazole, a poorly water soluble base-type drug, was studied. A synergisticeffect was found in ternary systems, largely more effective than correspondingbinary complexes and salts. Moreover, the presence of a third component madeeffective the use of γ-CD, which had no solubilizing power in binarysystems. The solubilizing efficiency of multicomponent systems was not relatedto the solubilities of the corresponding salts or binary complexes. Phase-solubility analysis at different temperatures was also used to investigate the interaction of econazole with cyclodextrins, alone or in the presence of hydroxyacid. The best 1 : 1 : 1 molar ratio system was that with α-CD and malic acid which showed the best solubilizing power and the highest stability constant of the ternary complex.Ternary α-CD products, prepared by co-grinding, co-evaporation or colyophilization, were characterized by Differential Scanning Calorimetry and tested for dissolution properties. The higher solubilizing properties of multicomponent systems were reflected in better drug dissolution rates from their solid systems.
Similar content being viewed by others
References
K. Uekama, F. Hirayama, and T. Irie: Chem. Rev. 98, 2045 (1998).
D. Duchene: Cyclodextrins and their Industrial Uses, Editions De Santé, Paris (1987).
J. Szejtli: Cyclodextrin Technology, Kluwer, Dordrecht, The Netherlands (1988).
P. Chiesi, P. Ventura, M. Pasini, J. Szejtli, M. Vikmon, and E. Redenti: PCT Int. Appl. 33 pp. (1994).
E. Fenyvesi, M. Vikmon, J. Szeman, J. Szejtli, P. Ventura, and M. Pasini: Proc. 7th Int. Symp. Cyclodextrins, T. Osa (ed.), Academic Societies Japan, Tokyo (1994), pp. 414-418.
M. Vikmon, J. Szeman, J. Szejtli, M. Pasini, E. Redenti, and P. Ventura: Proc. 7th Int. Symp. Cyclodextrins, T. Osa (ed.), Academic Societies Japan, Tokyo (1994), pp. 480-483.
M.T. Esclusa-Diaz, M. Gayo-Otero, M.B. Pérez-Marcos, J.L. Vila-Jato, and J.J. Torres-Labandeira: Int. J. Pharm. 142, 183 (1996).
A.M. Le Ray, V. Bourges, J.M. H. Robert, P. Richomme, and C. Merle: Proc. 8th Int. Symp. Cyclodextrins, Kluwer Academic Publishers (1996), pp. 249-252.
Martindale, The Extra Pharmacopoeia 31st Edition, J. E. F. Reynolds (ed.), Royal Pharm. Soc., Pharmaceutical Press, London (1996), p. 404.
P. Mura, A. Liguori, G. Bramanti, G.P. Bettinetti, E. Campisi, and E. Faggi: Eur. J. Pharm. Biopharm. 38, 119 (1992).
L.J. Bononi: Eur. Pat. Appl. 19 p. (1988).
M. Pedersen, M. Edelsten, V.F. Nielsen, A. Scarpellini, S. Skytte, and C. Slot: Int. J. Pharm. 90, 247 (1993).
G. Piel, M. Fillet, B. Evrard, J. Crommen, and L. Delattre: Conference on Phamaceutical Application of Cyclodextrins, Lawrence, Kansas, USA (1997).
M. Pedersen, S. Bjerregaard, J. Jacobsen, A. Larsen Rommelmayer, and A. Mehlsen Sorensen: Int. J. Pharm. 165, 57 (1998).
M. Pedersen, J. Jacobsen, A. Larsen Rommelmayer, and A. Mehlsen Sorensen: Drug Dev. Ind. Pharm. 25, 463 (1999).
P. Mura, G. Franchi, M.T. Faucci, A. Manderioli, and G. Bramanti: Proc. 9th Int. Symp. Cyclodextrins, Kluwer Academic Publishers (1999), pp. 375-378.
T. Higuchi and K. Connors: Phase Solubility Techniques (Advances in Analytical Chemistry and Instrumentation), C. Reilly (ed.), Wiley Interscience, New York (1965), pp. 117-212.
K.A. Khan: J. Pharm. Pharmacol. 27, 48 (1975).
L. Szente, J. Szejtli, M. Vikmon, J. Szeman, E. Fenyvesi, M. Pasini, E. Redenti, and P. Ventura: Proc. 1st World Meeting APGI/APV (1995), pp. 579-580.
J. Szeman, M. Vikmon, J. Szejtli, M. Pasini, and P. Ventura: Proc. 7th Int. Symp. Cyclodextrins, T. Osa (ed.), Academic Societies Japan, Tokyo (1994), pp. 266-269.
A. Gerlócezy, A. Vikmon, J. Szejtli, E. Redenti, and P. Ventura: Proc. 9th Int. Symp. Cyclodextrins, Kluwer Academic Publishers (1999), pp. 277-280.
M.T. Faucci, F.Melani, and P.Mura: Proc. 8th Int. Meeting on Recent Development in Pharmaceutical Analysis (1999), p. 61.
I. Orienti, A. Fini, V. Bertasi, and V. Zecchi: Eur. J. Pharm. Biopharm. 37, 110 (1991).
R. Krishnamoorthy, and A.K. Mitra: Int. J. Pharm. Advances 1, 330 (1996).
A. Muñoz de la Peña, T.T. Ndou, J.B. Zung, K.L. Greene, D.H. Live, and I.M. Warner: J. Am. Chem. Soc. 113, 1572 (1991).
A. Ueno, K. Takahushi, Y. Hino, and T. Osa: J. Chem. Soc., Chem. Commun. 194 (1981).
K.H. Kim, M.J. Frank, and N.L. Henderson: J. Pharm. Sci. 74, 283 (1985).
O.I. Corrigan and T. Stanley: J. Pharm. Pharmacol. 34, 621 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mura, P., Faucci, M.T., Manderioli, A. et al. Multicomponent Systems of Econazole with Hydroxyacids and Cyclodextrins. Journal of Inclusion Phenomena 39, 131–138 (2001). https://doi.org/10.1023/A:1008114411503
Issue Date:
DOI: https://doi.org/10.1023/A:1008114411503