Abstract
Purpose. To elucidate the solution conditions that confer stability of aqueous IL-1R using differential scanning calorimetry (DSC).
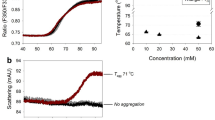
Methods. Optimal pH conditions were determined by monitoring degradation products encountered during accelerated studies (at elevated temperatures) using SDS-PAGE. At the pH optimum, DSC screened for excipients that enhanced thermal stability by shifting the Tm to higher values. Using SEC the relationship between thermal unfolding and stability was investigated by considering if lower Tm's in the presence of preservatives correlated with degradation products at 37°C over time. The degree of aggregation relative to that of a control determined the level of stability achieved.
Results. Circular dichroism (CD) measurements confirmed molecular modeling studies showing IL-1R to be about 39% β-sheet. Two major transitions characterized the DSC data with Tm's observed near 47°C and 66°C. Among 21 excipients screened, NaCl exhibited the greatest stabilizing influences based on shifting the low temperature transition to 53°C. The low temperature transition was later found to comprise two transitions, yielding a total of three melting transitions for IL-1R. High Tm's arising from the presence of preservatives correlated with the order of stability (i.e., 0.065% phenol > 0.1% m-Cresol > 0.9% benzyl alcohol).
Conclusions. The three melting transitions are consistent in origin with the cooperative unfolding of three unique immunoglobulin-like domains of IL-1R. Optimal stability was achieved in 20 mM sodium citrate at pH 6 with sufficient NaCl to attain the tonicity of human serum. A correlation between the predicted ranking of stability and the extent of aggregation was demonstrated using DSC.
Similar content being viewed by others
REFERENCES
J. J. Oppenheim, E. J. Kovacs, K. Matsushima, and S. K. Durum. Immunol. Today 7:45–56 (1986).
J. E. Sims, R. B. Acres, C. E. Grubin, C. J. McMahan, J. M. Wignall, C. J. March, and S. K. Dower. Proc. Natl. Acad. Sci. USA 86:8946–8950 (1990).
A. F. Williams. Immunol. Today 8:298–303 (1987).
J. A. Shrier, R. A. Kenley, R. Williams, R. J. Corcoran, Y. Kim, R. P. Northey, Jr., D. D'Augusta, and M. Huberty. Pharm. Res. 10:933–944 (1993).
T. H. Nguyen. In J. L. Cleland and R. Langer (eds.) Formulation and Delivery of Proteins and Peptides, American Chemical Society, Washington, D.C., 1994, pp. 59–71.
P. K. Tsai, D. B. Volkin, J. M. Dabora, K. C. Thompson, M. W. Bruner, J. O. Gress, B. Matuszewska, M. Keogan, J. V. Bondi, and C. R. Middaugh. Pharm. Res. 10:649–659 (1993).
S. Yoshioka, Y. Aso, K. Izutsu, and T. Terao. Pharm. Res. 10:687–691 (1993).
T. T. Herskovits, and H. Jaillet. Science 163:282–285 (1969).
L. R. De Young, K. A. Dill, and A. L. Fink. Biochemistry 32:3877–3886 (1993).
D. B. Volkin, P. K. Tsai, J. M. Dabora, J. O. Gress, C. J. Burke, R. J. Linhardt, and C. R. Middaugh. Arch. Biochem. Biophys. 300:30–41 (1993).
B. Chen, T. Arakawa, C. F. Morris, W. C. Kenney, C. M. Wells, and C. G. Pitt. Pharm. Res. 11:1581–1587 (1994).
A. Mitraki, J. M. Betton, M. Desmadril, and J. M. Yon. Eur. J. Biochem. 163:29–34 (1987).
S. J. Wearne, and T. E. Creighton. Proteins 5:8–12 (1989).
M. F. Powell. In J. L. Cleland and R. Langer (eds.) Formulation and Delivery of Proteins and Peptides, American Chemical Society, Washington, D. C., 1994, pp. 100–117.
B. S. Chang, C. S. Randall, and Y. S. Lee. Pharm. Res. 10:1478–1483 (1993).
A. Perczel, M. Hollosi, G. Tusnady, and G. D. Fasman. Protein Eng. 4:669–679 (1991).
A. Perczel, K. Park, and G. D. Fasman. Anal. Biochem. 203:83–93 (1992).
S. Srinivasan, C. J. March, and S. Sudarsanam. Protein Sci. 2:277–289 (1993).
M. Oh-eda, M. Hasegawa, K. Hattori, H. Kuboniwa, T. Kojima, T. Orita, K. Tomonou, T. Yamazaki, and N. Ochi. J. Biol. Chem. 265:11432–11435 (1990).
M. F. Powell, T. Stewart, L. Otvos, Jr., L. Urge, F. C. A. Gaeta, A. Sette, T. Arrhenius, D. Thomson, K. Soda, and S. M. Colon. Pharm. Res. 10:1268–1273 (1993).
T. Asakura, K. Adachi, and E. Schwartz. J. Biol. Chem. 253:6423–6425 (1978).
L. L. Lee, and J. C. Lee. Biochemistry 26:7813–7819 (1987).
W. R. Gombotz, S. C. Pankey, D. Phan, R. Drager, K. Donaldson, K. P. Antonsen, A. S. Hoffman, and H. V. Raff. Pharm. Res. 11:624–632 (1994).
S. Damodaran and J. E. Kinsella. J. Biol. Chem. 255:8503–8508 (1980).
P. L. Privalov, N. N. Khechinashvilli, and B. P. Atanasov. Biopolymers 10:1865–1890 (1971).
P. L. Privalov. Adv. Protein Chem. 33:167–241 (1979).
C. Q. Hu, and J. M. Sturtevant. Biochemistry 26:178–182 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Remmele, R.L., Nightlinger, N.S., Srinivasan, S. et al. Interleukin-1 Receptor (IL-1R) Liquid Formulation Development Using Differential Scanning Calorimetry. Pharm Res 15, 200–208 (1998). https://doi.org/10.1023/A:1011902215383
Issue Date:
DOI: https://doi.org/10.1023/A:1011902215383