Abstract
Purpose. Orthorhombic crystals of paracetamol exhibit good technological properties during compression. The purpose of this study was to investigate the compression behavior of this substance and to compare it to that of monoclinic paracetamol. From the crystal structure, it could be hypothesized that sliding planes are present in the orthorhombic form, and could be responsible for an increase in crystal plasticity.
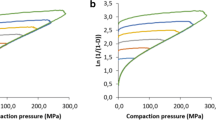
Methods. Compression of pure orthorhombic or monoclinic paracetamol tablets was carried out on a fully instrumented single punch machine. Data was used to establish Heckel's profiles. Images of compressed crystals were obtained by scanning electron microscopy.
Results. Tabletability of the orthorhombic crystals was far better than that of the monoclinic ones, and capping was not observed even at high compression pressure. Compared to the monoclinic form, orthorhombic paracetamol exhibited greater fragmentation at low pressure, increased plastic deformation at higher pressure, and lower elastic recovery during decompression. Plastic behavior was confirmed by SEM - micrographs showing that crystals folded under pressure. A compactibility study showed that the nature of interparticle bonds was similar for both polymorphs, the number of bonds being greater for orthorhombic paracetamol.
Conclusions. Unlike the monoclinic form, orthorhombic paracetamol is suitable for the direct compression process. The crystalline structure accounts for its better compression behavior, because of the presence of sliding planes.
Similar content being viewed by others
REFERENCES
M. Duberg and C. Nyström. Studies on Direct Compression of Tablets. XVII. Porosity-pressure curves for the characterization of volume reduction mechanisms in powder compression. Powder Technol. 46:67–75 (1994).
D. Train. An investigation into the compaction of powder. J. Pharm. Pharmacol. 8:745–761 (1956).
J. S. M. Garr and M. H. Rubinstein. An investigation into the capping of paracetamol at increasing speeds of compression. Int. J. Pharm. 72:117–122 (1991).
R. Hüttenrauch. New concepts in pharmaceutics. Labo-Pharma-Probl. Tech. 31:644–655 (1983).
P. York. Crystal engineering and particle design for the powder compaction process. Drug Dev. Ind. Pharm. 18:677–721 (1992).
J. M. Fachaux, A. M. Guyot-Hermann, J. C. Guyot, P. Conflant, M. Drache, S. Veesler, and R. Boistelle. Pure paracetamol for direct compression. Part I. Development of sintered—like crystals of paracetamol. Powder Technol. 82:123–128 (1995).
A. Ettabia, E. Joiris, A. M. Guyot-Hermann, and J. C. Guyot. Preparation of a pure paracetamol for direct compression by spherical agglomeration. Pharm. Ind. 59:625–631 (1997).
P. Di Martino, A. M. Guyot-Hermann, P. Conflant, and J. C. Guyot. A new pure paracetamol for direct compression: the orthorhombic form Int. J. Pharm. 128:1–8 (1996).
M. Haïsa, S. Kashino, and H. Maeda. The orthorhombic form of p-hydroxyacetanilide. Acta Cryst. B30:2510–2513 (1974).
M. Haïsa, S. Kashino, R. Kawai, and H. Maeda. The monoclinic form of p-hydroxyacetanilide. Acta Cryst. B32:1283–1285 (1976).
H. Hess. Tablets under the microscope. Pharm. Technol. 9:37–58 (1978)
P. Di Martino, P. Conflant, M. Drache, J. P. Huvenne, and A. M. Guyot-Hermann. Preparation and physical characterization of Forms II and III of paracetamol. J. Thermal Anal. 48:447–458 (1997).
C. Lefebvre, F. Guillaume, R. Bouché, R. Bouaziz, and J. C. Guyot. Etude de différentes formes polymorphes du méprobamate. 5th International Conference On Pharmaceutical Technology. Paris. I: 211–221 (1989).
M. J. Juslin and T. P. Paronen. On the accuracy of displacement measurements by instrumented single-punch machines. J. Pharm. Pharmacol. 32:796–798 (1980).
J. T. Fell and J. M. Newton. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 5:688–691 (1970).
N. A. Armstrong and R. F. Haines-Nutt. Elastic recovery and surface area changes in compacted powder systems. Powder Technol. 9:287–290 (1974).
R. W. Heckel. Density—pressure relationships in powder compaction. Trans. Metall. Soc. AIME, 221:661–675 (1961).
E. Doelker. Assessment of powder compaction. In D. Chulia, M. Deleuil and Y. Pourcelot (eds), Powder technology and pharmaceutical processes, Elsevier, Amsterdam, 1994, pp. 403–471.
M. Eriksson and G. Alderborn. The effect of original particle size and tablet porosity on the increase in tensile strength during storage of sodium chloride tablets in a dry atmosphere. Int. J. Pharm. 113:199–207 (1995).
P. Paronen and J. Ilkka. Porosity-pressure functions. In G. Alderborn and C. Nyström (eds), Pharmaceutical powder compaction technology, Marcel Dekker, New York, 1996, pp. 55–75.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Joiris, E., Martino, P.D., Berneron, C. et al. Compression Behavior of Orthorhombic Paracetamol. Pharm Res 15, 1122–1130 (1998). https://doi.org/10.1023/A:1011954800246
Issue Date:
DOI: https://doi.org/10.1023/A:1011954800246