Abstract
Purpose. To better understand the nature of drug-excipient interactions we have studied the phase behavior of amorphous binary and ternary mixtures of citric acid, indomethacin and PVP, as model systems.
Methods. We have prepared amorphous mixtures by co-melting or coprecipitation from solvents, and have measured glass transition temperatures with differential scanning calorimetry.
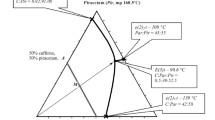
Results. Citric acid and indomethacin in the amorphous state are miscible up to 0.25 weight fraction of citric acid, equivalent to about 2 moles of citric acid and 3 moles of indomethacin. Phase separation, as reflected by two Tg values, occurs without crystallization leading to a saturated citric acid-indomethacin amorphous phase and one essentially containing only citric acid. PVP-citric acid and PVP-indomethacin form non-ideal miscible systems at all compositions. A ternary system containing 0.3 weight fraction of PVP produces a completely miscible system at all citric acid-indomethacin compositions. The use of 0.2 weight fraction of PVP, however, only produces miscibility up to a weight fraction of 0.4 citric acid relative to indomethacin. The two phases above this point appear to contain citric acid in PVP and citric acid in indomethacin, respectively.
Conclusions. Two components of an amorphous solid mixture containing citric acid and indomethacin with limited solid state miscibility can be solublized as an amorphous solid phase by the addition of moderate levels of PVP.
Similar content being viewed by others
REFERENCES
C. Ahlneck and G. Zografi. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. J. Pharm. Sci. 62:87-95 (1990).
B. C. Hancock and G. Zografi. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 86:1-12 (1997).
Q. Lu and G. Zografi. Properties of citric acid at the glass transition. J. Pharm. Sci. 86:1374-1378 (1997).
M. Yoshioka, B. C. Hancock, and G. Zografi. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J. Pharm. Sci. 83:1700-1705 (1994).
L. S. Taylor and G. Zografi. Spectroscopic characteristics of interaction between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res. 14:1691-1698 (1997).
S. L. Shamblin, E. Y. Huang, and G. Zografi. The effects of co-lyophilized polymeric additives on the glass transition and crystallization of amorphous sucrose. J. Thermal Anal. 47:1567-1579 (1996).
B. C. Hancock and G. Zografi. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm. Res. 11:471-477 (1994).
C. A. Oksanen and G. Zografi. The relationship between the glass transition temperature and water vapor absorption by poly(vinyl-pyrrolidone). Pharm. Res. 7:654-657 (1990).
M. Yoshioka, B. C. Hancock, and G. Zografi. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipitates. J. Pharm. Sci. 84:983-986 (1995).
M. Gordon and J. S. Taylor. Ideal copolymers and the second-order transition of synthetic rubbers, I. non-crystalline copolymers. J. Appl. Chem. 2:493-500 (1952).
R. Simha and R. F. Boyer. On a general relation involving the glass transition temperature and coefficient of expansion of polymers. J. Chem. Phys. 37:1003-1007 (1962).
H. A. Schneider. Glass transition behavior of compatible polymer blends. Polymer 30:771-779 (1989).
S. L. Shamblin, L. S. Taylor, and G. Zografi. The mixing behavior of co-lyophilized binary systems. J. Pharm. Sci. 87:694-701 (1998).
P. C. Painter, J. F. Graf, and M. M. Coleman. Effect of hydrogen bonding on the enthalpy of mixing and the composition dependence of the glass transition temperature in polymer blends. Macromolecules 24:5630-5638 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lu, Q., Zografi, G. Phase Behavior of Binary and Ternary Amorphous Mixtures Containing Indomethacin, Citric Acid, and PVP. Pharm Res 15, 1202–1206 (1998). https://doi.org/10.1023/A:1011983606606
Issue Date:
DOI: https://doi.org/10.1023/A:1011983606606