Abstract
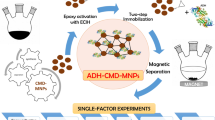
Yeast alcohol dehydrogenase (YADH) was immobilized covalently on Fe3O4 magnetic nanoparticles (10.6 nm) via carbodiimide activation. The immobilization process did not affect the size and structure of magnetic nanoparticles. The YADH-immobilized magnetic nanoparticles were superparamagnetic with a saturation magnetization of 61 emu g−1, only slightly lower than that of the naked ones (63 emu g−1). Compared to the free enzyme, the immobilized YADH retained 62% activity and showed a 10-fold increased stability and a 2.7-fold increased activity at pH 5. For the reduction of 2-butanone by immobilized YADH, the activation energies within 25–45 °C, the maximum specific activity, and the Michaelis constants for NADH and 2-butanone were 27 J mol−1, 0.23 mol min−1 mg−1, 0.62 mM, and 0.43 M, respectively. These results indicated a structural change of YADH with a decrease in affinity for NADH and 2-butanone after immobilization compared to the free enzyme.
Similar content being viewed by others
References
Chen DH,Wu SH (2000) Synthesis of nickel nanoparticles in waterin-oil microemulsions. Chem. Mater. 12: 1354-1360.
Chen DH,Chen HH,Huang TC (1995) Kinetic behavior of yeast alcohol dehydrogenase in AOT/isooctane reverse micelles. J. Chem. Tech. Biotechnol. 64: 217-224.
Cochrane FC,Petach HH,Henderson W (1996) Application of tris(hydroxymethyl)phosphine as a coupling agent for alcohol dehydrogenase immobilization. Enzyme Microbiol. Technol. 18: 373-378.
Dresco PA,Zaitsev VS,Gambino RJ,Chu B (1999) Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir 15: 1945-1951.
Halling PJ,Dunnill P (1980) Magnetic supports for immobilized enzymes and bioaffinity adsorbents. Enzyme Microbiol. Technol. 2: 2-10.
Hummel W,Kula MR (1989) Dehydrogenases for the synthesis of chiral compounds. Eur. J. Biochem. 184: 1-13.
Jones JB (1986) Enzymes in organic synthesis. Tetrahedron 42: 3351-3403.
Kondo A,Fukuda H (1997) Preparation of thermo-sensitive magnetic hydrogel microspheres and application to enzyme immobilization. J. Ferment. Bioeng. 84: 337-341.
Löster K,Seidel S,Kirstein D,Schneider F,Noll F (1992) Novel antibody coating of a magnetizable solid phase for use in enzyme immunoassays. J. Immunol. Meth. 148: 41-47.
Mehta RV,Upadhyay RV,Charles SW,Ramchand CN (1997) Direct binding of protein to magnetic particles. Biotechnol. Technol. 11: 493-496.
Ooshima H,Genko Y,Harano Y (1981) Stability of immobilized yeast alcohol dehydrogenase. Biotechnol. Bioeng. 23: 2851-2862.
Orlich B,Berger H,Lade M,Schomäcker R (2000) Stability and activity of alcohol dehydrogenases in w/o-microemulsions: enantioselective reduction including cofactor regeneration. Biotechnol. Bioeng. 70: 638-646.
Roath S (1993) Biological and biomedical aspects of magnetic fluid technology. J. Magn. Magn. Mater. 122: 329-334.
Schöpp W,Grunow M (1986) Immobilization of yeast ADH by adsorption onto polyaminomethylstyrene. Appl. Microbiol. Biotechnol. 24: 271-276.
Schütt W,Grüttner C,Häfeli U,Zborowski M,Teller J,Putzar H,Schümichen C (1997) Applications of magnetic targeting in diagnosis and therapy-possibilities and limitations: a mini-review. Hybridoma 16: 109-117.
Shinkai M,Honda H,Kobayashi T (1991) Preparation of fine magnetic particles and application for enzyme immobilization. Biocatalysis 5: 61-69.
Williams AK,Hupp JT (1998) Sol-gel-encapsulated alcohol dehydrogenase as a versatile, environmentally stabilized sensor for alcohols and aldehydes. J. Am. Chem. Soc. 120: 4366-4371.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, MH., Chen, DH. Immobilization of yeast alcohol dehydrogenase on magnetic nanoparticles for improving its stability. Biotechnology Letters 23, 1723–1727 (2001). https://doi.org/10.1023/A:1012485221802
Issue Date:
DOI: https://doi.org/10.1023/A:1012485221802