Abstract
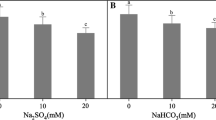
White spruce [Picea glauca (Moench) Voss] seedlings were inoculated with Hebeloma crustuliniforme and treated with 25 mM NaCl to examine the effects of salinized soil and mycorrhizae on root hydraulic conductance and growth. Mycorrhizal seedlings had significantly greater shoot and root dry weights, number of lateral branches and chlorophyll content than non-mycorrhizal seedlings. Salt treatment reduced seedling growth in both non-mycorrhizal and mycorrhizal seedlings. However, needles of salt-treated mycorrhizal seedlings had several-fold higher needle chlorophyll content than that in non-mycorrhizal seedlings treated with salt. Mycorrhizae increased N and P concentrations in seedlings. Na levels in shoots and roots of salt-treated mycorrhizal seedlings were significantly lower and root hydraulic conductance was several-fold higher than in non-mycorrhizal seedlings. A reduction of about 50% in root hydraulic conductance of mycorrhizal seedlings was observed after removal of the fungal hyphal sheath. Transpiration and root respiration rates were reduced by salt treatments in both groups of seedlings compared with the controls, however, both transpiration and respiration rates of salt-treated mycorrhizal seedlings were as high as those in the non-mycorrhizal seedlings that had not been subjected to salt treatment. The reduction of shoot Na uptake while increasing N and P absorption and maintaining high transpiration rates and root hydraulic conductance may be important resistance mechanisms in ectomycorrhizal plants growing in salinized soil.
Similar content being viewed by others
References
Alam S M 1994 Nutrient uptake by plants under stress conditions. In Handbook of Plant Stress. Ed M Pessakakli. pp 227–246. Marcel Dekker, New York.
Al-Karki G N 2000 Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 10, 51–54.
Allen M J 1992 Mycorrhizal Functioning. Chapman and Hall, New York.
Azcon R and El-Atrash F 1997 Influence of arbuscular mycorrhizae and phosphorus fertilization on growth, nodulation and N2 (N-15) in Medicago sativa at four salinity levels. Biol. Fertil. Soils 24, 81–86.
Bernstein L 1975 Effect of salinity and sodicity on plant growth. Am. Rev. Phytopathol. 13, 295–311.
Bolanose J A and Longstreth D J 1984 Salinity effect on water potential components and bulk elastic modulus of Alternantheria philoxeroides (Mart.) Griseb. Plant Physiol. 7 75, 281–284.
Carvajal M, Cerda A, and Martinez V 2000 Does calcium ameliorate the negative effect of NaCl on melon root water transport by regulating aquaporin activity? New Phytol. 145, 439–447.
Clarkson D T, Carvajal M, Henzler T, Waterhouse R N, Smyth A J, Cooke D T and Steudle E 2000 Root hydraulic conductance: diurnal aquaporin expression and the effect of nutrient stress. J. Exp. Bot. 51, 61–70.
Coleman M D, Bledsoe C S and Smit B A 1990 Root hydraulic conductivity and xylem sap levels of zeatin riboside and abscisic acid in ectomycorrhizal Douglas fir seedlings. New Phytol. 115, 275–284.
Epstein E 1972 Mineral nutrition of plants: principles and perspectives. John Wiley and Sons, Inc., London, 412 pp.
Graham J H and Syvertsen J P 1989 Vesicular-arbuscular mycorrhizas increase chloride concentration in citrus seedlings. New Phytol. 113, 29–36.
Greenway H and Munns R 1980 Mechanism of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 31, 149–190.
Hagemeyer J 1997 Salt. In Plant Ecophysiology. Ed M N V Prasad. pp 173–206. John Wiley & Sons, New York.
Hampp R, Wiese J, Mikolajewski S and Nehls U 1999 Biochemical and molecular aspects of C/N interaction in ectomycorrhizal plants. Plant Soil 215, 103–113.
Harley J L 1989 The significance of mycorrhiza. Mycol. Res. 92, 129–139.
Harley J L and Smith S E 1983 Mycorrhizal Symbiosis. Academic Press, New York.
Hartmond U, Schaesberg N, Graham J K and Syvertsen J P 1987 Salinity and flooding stress effects on mycorrhizal and nonmycorrhizal citrus rootstock seedlings. Plant Soil 104, 37–43.
Jentschke G, Brandes B, Kuhn A J, Schroder W H, Becker J S and Godbold D L 2000 The mycorrhizal fungus Paxillus involutus transport magnesium to Norway spruce seedlings. Evidence from stable isotope labeling. Plant Soil 220, 243–246.
Kamaluddin M and Zwiazek J J 2001 Metabolic inhibition of root water flow in red-osier dogwood (Cornus stolonifera) seedlings. J. Exp. Bot. 52: 739–745.
Koied R 1985 The effects of VA mycorrhizal infection and phosphorus status on sunflower stomatal properties. J. Exp. Bot. 36, 1087–1098.
Kuiper P J C 1984 Functioning of plant cell membranes under saline conditions. In Salinity Tolerance in Plants. Ed R C Staples and G H Toenniessen. pp 77–91. Wiley Interscience, New York, NY.
Martinez-Ballesta M D, Martinez V and Carvajal M 2000 Regulation of water channel activity in whole roots and in protoplasts from roots of melon plants grown under saline conditions. Aust. J. Plant Physiol. 27, 685–691.
Mason P A 1980 Aseptic synthesis of sheathing (ecto-) mycorrhizas. In Tissue Culture Methods for Plant Pathologists. Eds D S Ingram and J P Helgeson. pp 173–178. Blackwell Sci. Publ., Oxford.
Molina R 1982 Use of the ectomycorrhizal fungus Laccaria laccata in forestry. I. Consistency between isolates in effective colonization of containerized conifer seedlings. Can. J. For. Res. 12, 469–473.
Munns R 1993 Physiological responses limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ. 16, 15–24.
Park J L, Lindermann R G and Black C H 1983 The role of ectomycorrhizas in drought tolerance of Douglas fir seedlings. New Phytol. 95, 83–95.
Perry D A, Molina R and Amaranthus M P 1987 Mycorrhizae, mycorrhizospheres, and reforestation: current knowledge and research needs. Can. J. For. Res. 17, 929–940.
Quoreshi A M and Timmer V R 1998 Exponential fertilization increases nutrient uptake and ectomycorrhizal development of black spruce seedlings. Can. J. For. Res. 28, 674–682.
Read D J 1991 Mycorrhizas in ecosystem. Experimentia 47, 376–391.
Renault S, Lait C, Zwiazek J J and MacKinnon M 1998 Effect of high salinity tailings waters produced from gypsum treatment of oil sand tailings on plants of the boreal forest. Environ. Pollut. 102, 177–184.
Renault S, Paton E, Nilsson G, Zwiazek J J and MacKinnon M D 1999 Responses of boreal plants to high salinity oil sands tailings water. J. Environ. Qual. 28, 1957–1962.
Richard J E 1993 Chemical characterization of plant tissues. In Soil sampling and methods of analysis. Ed M R Carter. pp 115–139. Lewis Publishers, Boca Raton, FL.
Sands R Fiscus E L and Reid C PP 1982 Hydraulic properties of pine and bean roots with varying degrees of suberization, vascular differentiation and mycorrhizal infection. Aust. J Plant Physiol. 9, 559–569.
Schenck N C 1982 Methods and principles of mycorrhizal research. Am. Phytopath. Soc. St. Paul, Minnesota, MN.
Sestak Z, Catsky J and Jarvis P G 1971 Plant Photosynthetic Production. W Junk, The Hague, 391 pp.
Shaw C H, Molina R and Walden J 1982 Development of ectomycorrhizae following inoculation of containerized Sitka and white spruce seedlings. Can. J. For. 12, 191–195.
Smith S E and Read D J 1997. Mycorrhizal Symbiosis. Academic Press, New York.
Steudle E and Peterson C A 1998 How does water get through roots? J. Exp. Bot. 49, 775–788.
Tyree M T, Patino S, Bennink J and Alexander J 1995 Dynamic measurements of root hydraulic conductance using a highpressure flowmeter in the laboratory and field. J. Exp. Bot. 46: 83–94.
Wallander H and Nylund J E 1992 Effects of excess nitrogen on carbohydrate concentration and mycorrhizal development of Pinus sylvestris L. seedlings. New Phytol. 119, 405–411.
Wan X and Zwiazek J J 1999 Mercuric chloride effects on root water transport in aspen seedlings. Plant Physiol. 121, 939–946.
Wan X and Zwiazek J J 2001 Root water flow and leaf stomatal conductance in aspen (Populus tremuloides) seedlings treated with ABA. Planta 213, 741–747.
Wan X, Landhäusser S M, Zwiazek J J and Lieffers V J 1999. Rootwater flowand growth of aspen (Poulus tremuloides) at low temperature. Tree Physiol. 19, 879–884.
Wan X, Zwiazek J J, Lieffers V J and Landhäusser S M 2001 Hydraulic conductance in aspen (Populus tremuloides) seedlings exposed to low root temperature. Tree Physiol. 21, 691–696.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muhsin, T.M., Zwiazek, J.J. Colonization with Hebeloma crustuliniforme increases water conductance and limits shoot sodium uptake in white spruce (Picea glauca) seedlings. Plant and Soil 238, 217–225 (2002). https://doi.org/10.1023/A:1014435407735
Issue Date:
DOI: https://doi.org/10.1023/A:1014435407735