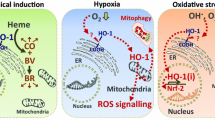
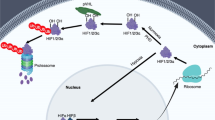
Abstract
Modern methods of cell and molecular biology, augmented by molecular technology, have great potential for helping to unravel the complex mechanisms of various diseases. They also have the potential to help us try to dissect the events which follow the altered physiological conditions. Thus, there is every reason to believe that some of the potential mechanisms will be translated sooner or later into the clinic. Heme oxygenase (HO)-related mechanisms play an important role in several aspects of different diseases. In the past several years, significant progress has been made in our understanding of the function and regulation of HO. The objective of this article is to review current knowledge relating to the importance of HO mechanism in various diseases including myocardial ischemia/reperfusion, hypertension, cardiomyopathy, organ transplantation, endotoxemia, lung diseases, and immunosuppression. The morbidity and mortality of these diseases remain high even with optimal medical management. Furthermore, in this review, we consider various factors influencing the HO system and finally assess current pharmacological approaches to their control.
Similar content being viewed by others
References
Tenhunen R, Marver HS, Schmid R: Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 244: 6388–6394, 1969
Tenhunen R, Ross ME, Marver HS, Schmid R: Reduced nicotinamideadenine dinucleotide phosphate dependent biliverdin reductase: Partial purification and characterization. Biochemistry 9: 298–303, 1970
Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH: Carbon monoxide: A putative neural messenger. Science 259: 381–384, 1993
Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH: Heme oxygenase 2: Endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci USA 93: 795–798, 1996
Keyse SM, Tyrrell RM: Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA 86: 99–103, 1989
Ewing JF, Maines MD: Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: Heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci USA 88: 5364–5368, 1991
Muller RM, Taguchi H, Shibahara S: Nucleotide sequence and organization of the rat heme oxygenase gene. J Biol Chem 262: 6795–6802, 1987
McCoubrey WK, Maines MD: The structure, organization, and differential expression of the gene encoding rat heme oxygenase-2. Gene 139: 155–161, 1994
Kutty RK, Kutty G, Rodriguez IR, Chader GJ, Wiggert B: Chromosomal localization of the human heme oxygenase genes: Heme oxygenase-1 (HEMOX1) maps to chromosome 22q12 and heme oxygenase-2 (HEMOX2) maps to chromosome 16p13.3. Genomics 20: 513–516, 1994
Maines MD: The heme oxygenase system: A regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517–554, 1997
Brown SB: Stereospecific heme cleavage. A model for the formation of bile pigment isomers in vivo and in vitro. Biochem J 159: 23–27, 1976
Sjostrand T: Endogenous formation carbon monoxide in man under normal and pathological conditions. J Clin Lab Invest 1: 201–210, 1949
Tenhunen R, Marver HS, Schmid R: The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61: 748–755, 1968
Kutty RK, Maines MD: Characterization of a NADH-dependent heme degrading system in ox heart mitochondria. Biochem J 246: 467–474, 1987
Guengerich FP: Destruction of heme and hemoproteins mediated by liver microsomal reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase. Biochemistry 17: 3633–3639, 1978
Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R: Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Phys 278: H643–651, 2000
Minowada G, Welch WJ: Clinical implications of the stress response. J Clin Invest 95: 3–12, 1995
Maines MD: Carbon monoxide: An emerging regulator of cGMP in the brain. Mol Cell Neurosci 4: 389–398, 1993
Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, Winslow RM: Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res 83: 568–577, 1998
Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of heme oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J 348: 615–619, 2000
Maulik N., Engelman DT, Watanabe M, Engelman RM, Rousou JA, Flack JE III, Deaton DW, Gorbunov NV, Elsayed NM, Kagan VE, Das DK: Nitric oxide/carbon monoxide. A molecular switch for myocardial preservation during ischemia. Circulation 94(suppl II): II398–II406, 1996
Sharma HS, Maulik N, Gho BC, Das DK, Verdouw PD: Coordinated expression of heme oxygenase-1 and ubiquitin in the porcine heart subjected to ischemia and reperfusion. Mol Cell Biochem 157: 111–116, 1996
Hangaishi M, Ishizaka N, Aizawa T, Kurihara Y, Taguchi J, Nagai R, Kimura S, Ohno M: Induction of heme oxygenase-1 can act protectively against cardiac ischemia/reperfusion in vivo. Biochem Biophys Res Commun 279: 582–588, 2000
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA: Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87: 1620–1624, 1990
Murphy BJ, Ladeorute KR, Short SM, Sutherland RM: The identification of heme oxygenase as a major hypoxic stress protein in Chinese hamster ovary cells. Br J Cancer 64: 69–73, 1991
Wang GL, Semenza GL: General involvement of hypoxia inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 90: 4304–4308, 1993
Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L: Hypoxiaresponsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med 182: 1683–1693, 1995
Tosaki A, Maulik N, Elliott GT, Blasig IE, Engelman RM, Das DK: Preconditioning of rat heart with monophosphoryl lipid A: A role of nitric oxide. J Pharmacol Exp Ther 285: 1274–1279, 1998
Csonka C, Varga E, Kovacs P, Ferdinandy P, Blasig IE, Szilvassy Z, Tosaki A: Heme oxygenase and cardiac function in ischemic/reperfused rat hearts. Free Rad Biol Med 27: 119–126, 1999
Pataki T, Bak I, Csonka C, Kovacs P, Varga E, Blasig IE, Tosaki A: Regulation of ventricular fibrillation by heme oxygenase in ischemic/reperfused hearts. Antiox Redox Signal 3: 125–134, 2001
Johnson RA, Lavesa M, Askari B, Abraham NG, Nasitetti A: A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension 25: 166–169, 1995
Lo WC, Jan CR, Chiang HT, Tseng JC: Modulatory effects of carbon monoxide on baroreflex activation in nucleus tractus solitarii of rats. Hypertension 35: 1253–1257, 2000
Morita T, Kourembanas S: Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest 96: 2676–2682, 1995
Johnson RA, Colombari E, Colombari DS, Lavesa M, Talman WT, Nasiletti A: Role of endogenous carbon monoxide in central regulation of arterial pressure. Hypertension 30: 962–967, 1997
Hartsfield CL, Alam J, Cook JL, Choi AM: Regulation of heme oxygenase-1 gene expression in vascular smooth muscle cells by nitric oxide. Am J Physiol 273: L980–L988, 1997
Loscalzo J, Welch G: Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis 38: 87–104, 1995
Beasley D, Schwartz JH, Brenner BM: Interleukin-1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest 87: 602–608, 1991
Durante W, Kroll MH, Christodaulides N, Peyton KJ, Schafer AL: Nitric oxide induces heme-oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res 80: 557–564, 1997
Morita T, Perrella MA, Lee ME, Kourembanas S: Smooth muscle cellderived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA 92: 1475–1479, 1995
Hansson GK, Geng YJ, Holm J, Hardhammar P, Wennmalm A, Jennische E: Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. J Exp Med 180: 733–738, 1994
Verbeuren TJ, Bonhomme E, Laubie M, Simonet S: Evidence for the induction of nonendothelial NO synthase in aortas of colesterol-fed rabbits. J Cardiovasc Pharmacol 21: 841–845, 1993
Albakri QA, Stuehr DJ: Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J Biol Chem 271: 5414–5421, 1996
Matheis G, Sherman MP, Buckberg GD, Haybron DM, Young HH, Ignarro LJ: Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am J Physiol 262: H616–H620, 1992
Liu H, Song D, Lee SS: Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am J Physiol 280: G68–G74, 2001
Raju VS, Imai N, Liang CS: Chamber-specific regulation of heme oxygenase-1 (heat shock protein 32) in right-sided congestive heart failure. J Mol Cell Cardiol 31: 1581–1589, 1999
Seki T, Naruse M, Naruse K, Yoshimoto T, Tanabe A, Seki M, Imaki T, Demura R, Demura R: Induction of heme oxygenase produces loadindependent cardioprotective effects in hypertensive rats. Life Sci 65: 1077–1086, 1999
Nath KA: Heme oxygenase-1: A redoubtable response that limits reperfusion injury in the transplanted adipose liver. J Clin Invest 104: 1485–1496, 1999
Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglienski JW: Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest 104: 1631–39, 1999
Lin Y, Soares MP, Sato K, Takigami K, Csizmadia E, Smith N, Bach FH: Accommodated xenografts survive in the presence of anti-donor antibodies and complement that precipitate rejection of naïve xenografts. J Immunol 163: 2850–2857, 1999
DeBruyne LA, Magee JC, Buelow R, Bromberg JS: Gene transfer of immunomodulatory peptides correlates with heme oxygenase-1 induction and enhanced allograft survival. Transplantation 69: 120–128, 2000
Parrillo JE: Pathogenetic mechanisms of septic shock. N Engl J Med 328: 1471–1477, 1993
Bone RC: Toward an epidemiology and natural history SIRS (systemic inflammatory response syndrome). JAMA 268: 3452–3455, 1992
Nathan CF: Secretory products of macrophages. J Clin Invest 79: 319–326, 1987
Epstein, FH: The role of interleukin-1 in disease. N Engl J Med 328: 106–107, 1993
Randow F, Synbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD: Mechanism of endotoxin desensitization involvement of interleukin 10 and transforming growth factor β. J Exp Med 181: 1887–1892, 1995
Yet SF, Pellacani A, Patterson C, Tan L, Folta SC, Foster L, Lee WS, Hsieh CM, Perrella MA: Induction of heme oxygenase-1 expression in vascular smooth muscle cells. A link to endotoxic shock. J Biol Chem 272: 4295–4301, 1997
Pellacani A, Wiesel P, Sharma A, Foster L, Huggins GS, Yet SF, Perella MA: Induction of heme oxygenase-1 during endotoxemia is down-regulated by transforming growth factor-β1. Circ Res 83: 396–403, 1998
Otterbein L, Chin BY, Otterbein SL, Lowe VC, Fessler HE, Choi AM: Mechanism of hemoglobin-induced protection against endotoxemia in rats: A ferritin-independent pathway. Am J Physiol 272: L268–L275, 1997
Otterbein L, Sylvester SL, Choi AM: Hemoglobin provides protection against lethal endotoxemia in rats: The role of heme oxygenase-1. Am J Respir Cell Mol Biol 13: 595–601, 1995
Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet SF, Lee ME, Perrella MA: Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation 102: 3015–3022, 2000
Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T: A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988
Ruetten H, Thiemermann C, Vane JR: Effects of the endothelin receptor antagonist, SB 209670, on circulatory failure and organ injury in endotoxic shock in the anesthetized rat. Br J Pharmacol 118: 198–204, 1996
Hollemberg SM, Piotrowski MJ, Parrillo JE: Nitric oxide synthase inhibition reverses arteriolar hyporesponsiveness to endothelin-1 in septic rats. Am J Physiol 272: R969–R974, 1997
Choi AM, Alam J: Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Resp Cell Mol Biol 15: 9–19, 1996
Zayasu K, Sekizawa K, Okinaga S, Yamaya M, Ohrui T, Sasaki H: Increased carbon monoxide in exhaled air of asthma patients. Am J Respir Crit Care Med 156: 1140–1143, 1997
Horvath I, Donnelly LE, Kiss A, Kharitonov SA, Barnes PJ: Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: A new marker of oxidative stress. Thorax 53: 668–672, 1998
Lim S, Groneberg D, Fischer A, Oates T, Caramori G, Mattos W, Adcook I, Barnes PJ, Chung KF: Expression of heme oxygenase isoenzymes 1 and 2 in normal and asthmatic airways. Am J Respir Crit Care Med 162: 1912–1918, 2000
Gutteridge JM, Mumby S, Quinlan GJ, Chung KF, Evans TW: Prooxidant iron is present in human pulmonary epithelial lining fluid: Implication for oxidative stress in the lung. Biochem Biophys Res Commun 220: 1024–1027, 1996
Willis D, Moore AR, Frederisk R, Willoughby DA: Heme oxygenase: A novel target for the modulation of the inflammatory response. Nat Med 2: 87–90, 1996
Genco CA, Odusanya BM, Brown G: Binding and accumulation of hemin in porphyromonas gingvalis are induced by hemin. Infect Immunol 62: 2885–2892, 1994
Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, Bach FH: Expression of heme oxygenase-1 (HO-1) can determine cardiac xenografts survival. Nat Med 4: 1073–1077, 1998
Bach FH, Ferran C, Soares M, Wrington CJ, Anrather J, Winkler H, Robson SC, Hancock WW: Modification of vascular responses in xenotransplantation: Inflammation and apoptosis. Nat Med 3: 944–948, 1997
Soares MP, Lin Y, Sato K, Stuhlmeier KM, Bach FH: Accommodation. Immunol Today 20: 434–437, 1999
Wang N, Lee JM, Soares MP, Csizmadia E, Robson SC, Smith N, Bach FH, Lin Y. Long-term survival of hamster hearts in presensitized rats. Transplant Proc 33: 747–758, 2001
Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP: Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol 166: 4185–4194, 2001
Miyatake T, Sato K, Takigami K, Koyamada N, Hancock WW, Bazin H, Latinne D, Bach FH, Soares MP. Complement-fixing elicited antibodies are a major component in the pathogenesis of xenografts rejection. J Immunol 160: 4114–4123, 1998
Bach FH, Robson SC, Ferran C, Winkler H, Millan MT, Stuhlmeier KM, Vanhove B, Blakely ML, van der Werf WJ, Hofer E: Endothelial cell activation and thromboregulation during xenograft rejection. Immunol Rev 141: 5–30, 1994
Woo J, Iyer S, Corneio MC, Mori N, Gao L, Sipos I, Maines M, Buelou R: Stress protein-induced immunosuppression: Inhibition of cellular immune effector functions following overexpression of heme oxygenase (HSP 32). Transpl Immunol 6: 84–93, 1998
Reeve VE, Tyrrell RM: Heme oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse. Proc Natl Acad Sci USA 96: 9317–9321, 1999
Rights and permissions
About this article
Cite this article
Tosaki, A., Das, D.K. The role of heme oxygenase signaling in various disorders. Mol Cell Biochem 232, 149–157 (2002). https://doi.org/10.1023/A:1014885014600
Issue Date:
DOI: https://doi.org/10.1023/A:1014885014600