Abstract
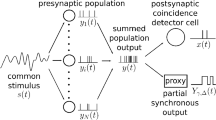
The response of leaky integrate-and-fire neurons is analyzed for periodic inputs whose phases vary with their spatial location. The model gives the relationship between the spatial summation distance and the degree of phase locking of the output spikes (i.e., locking to the periodic stochastic inputs, measured by the synchronization index). The synaptic inputs are modeled as an inhomogeneous Poisson process, and the analysis is carried out in the Gaussian approximation. The model has been applied to globular bushy cells of the cochlear nucleus, which receive converging inputs from auditory nerve fibers that originate at neighboring sites in the cochlea. The model elucidates the roles played by spatial summation and coincidence detection, showing how synchronization decreases with an increase in both frequency and spatial spread of inputs. It also shows under what conditions an enhancement of synchronization of the output relative to the input takes place.
Similar content being viewed by others
References
Adams JC, Mugnaini E (1987) Patterns of glutamate decarboxylase immunostaining in the feline cochlear nuclear complex studied with silver enhancement and electron microscopy. J. Comp. Neurol. 262: 375–401.
Anderson DJ (1973) Quantitative model for the effects of stimulus frequency upon synchronization of auditory nerve discharges. J. Acoust. Soc. Am. 54: 361–364.
Bose A, Booth V, Reece M (2000) A temporal mechanism for generating the phase precession of hippocampal place cells. J. Comput. Neurosci. 9: 5–30.
Brawer JR, Morest DK (1975) Relations between auditory nerve endings and cell types in the cat's anteroventral cochlear nucleus seen with the Golgi method and Nomarski optics. J. Comp. Neurol. 160: 491–506.
Brawer JR, Morest DK, Kane EC (1974) The neuronal architecture of the cochlear nucleus of the cat. J. Comp. Neurol. 155: 251–300.
Bruce IC, Irlicht LS, Clark GM (1998) A mathematical analysis of spatiotemporal summation of auditory nerve firings. Inform. Sciences 111: 303–334.
Buonomano DV, Mauk MD (1994) Neural network model of the cerebellum: Temporal discrimination and the timing of motor reponses. Neural Comput. 6: 38–55.
Burkitt AN, Clark GM (1999) Analysis of integrate-and-fire neurons: Synchronization of synaptic input and spike output in neural systems. Neural Comput. 11: 871–901.
Burkitt AN, Clark GM (2000) Calculation of interspike intervals for integrate-and-fire neurons with poisson distribution of synaptic inputs. Neural Comput. 12: 1789–1820.
Burkitt AN, Clark GM (2001) Synchronization of the neural response to noisy periodic synaptic input. Neural Comput. 13: 2639–2672.
Cai Y, Geisler CD (1996) Temporal patterns of the responses of auditory-nerve fibers to low-frequency tones. Hearing Res. 96: 83–93.
Cant NB, Morest DK (1979) Organization of the neurons in the anterior division of the anteroventral cochlear nucleus of the cat: Light-microscopic observations. Neurosci. 4: 1909–1923.
Cant NB, Morest DK (1979) The bushy cells in the anteroventral cochlear nucleus of the cat: Study with the electron-microscope. Neurosci. 4: 1925–1945.
Carney LH (1990) Sensitivities of cells in the anteroventral cochlear nucleus of cat to spatio-temporal discharge patterns across primary afferents. J. Neurophysiol. 64: 437–456.
Carney LH (1992) Modelling the sensitivity of cells in the anteroventral cochlear nucleus to spatiotemporal discharge patterns. Phil. Trans. R. Soc. Lond. B336: 403–406.
Carney LH (1994) Spatiotemporal encoding of sound level: Models for normal encoding and recruitment of loudness. Hearing Res. 76: 31–44.
Carney LH, Friedman M (1998) Spatiotemporal tuning of lowfrequency cells in the anteroventral cochlear nucleus. J. Neurosci. 18: 1096–1104.
Carr CE, Konishi M (1990) A circuit for detection of interaural time differences in the brain stem of the barn owl. J. Neurosci. 10: 3227–3246.
Clark GM (1996) Electrical stimulation of the auditory nerve: The coding of frequency, the perception of pitch, and the development of cochlear implant speech processing strategies for profoundly deaf people. Clin. Exp. Pharmacol. Physiol. 23: 766–776.
Clark GM (1999) Cochlear implants in the third millennium. Am. J. Otol. 20: 4–8.
Clark GM, Carter TD, Maffi CL, Shepherd RK (1995) Temporal coding of frequency: Neuron firing probabilities for acoustic and electrical stimulation of the auditory nerve. Ann. Otol. Rhinol. Laryngol. 104(Suppl. 166): 109–111.
Colburn HS, Han Y, Culotta CP (1990) Coincidence model of MSO responses. Hearing Res. 49: 335–346.
Cox DR, Lewis PAW (1966) The Statistical Analysis of Series of Events. Methuen, London.
Eggermont JJ, Aertsen AMJH, Johannesma PIM (1983) Quantitative characterisation procedure for auditory neurons based on the spectro-temporal receptive field. Hearing Res. 10: 167–190.
Evans EF (1981) The dynamic range problem: Place and time coding at the level of the cochlear nerve and nucleus. In: J Syka, LM Atkins, eds. Neuronal Mechanisms and Hearing. Plenum, New York. pp. 69–85.
Galambos R, Davis H (1943) The response of single auditory-nerve fibers to acoustic stimulation. J. Neurophysiol. 6: 39–57.
Gerstner W, Kempter R, van Hemmen JL, Wagner H (1996) A neuronal learning rule for sub-millisecond temporal coding. Nature 383: 76–78.
Goldberg JM, Brown PB (1969) Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: Some physiological mechanisms of sound localization. J. Neurophysiol. 32: 613–636.
Greenberg S (1996) Auditory processing of speech. In: NJ Lass, ed. Principles of Experimental Phonetics. Mosby, St. Louis. pp. 362–407.
Hohn N, Burkitt AN (2001) Shot noise in the leaky integrate-and-fire neuron. Phys. Rev. E 63: 031902.
Hubel D, Wiesel T (1962) Receptive fields, binocular interaction, and functional architecture in the cat's visual cortex. J. Physiol. 160: 106–154.
Jeffress LA (1948) A place theory of sound localization. J. Comp. Physiol. 41: 35–39.
Johnson DH (1980) The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. J. Acoust. Soc. Am. 68: 1115–1122.
Johnson DH, Swami A (1983) The transmission of signals by auditory-nerve fiber discharge patterns. J. Acoust. Soc. Am. 74: 493–501.
Joris PX, Carney LH, Smith PH, Yin TCT (1994a) Enhancement of neural synchronization in the anteroventral cochlear nucleus. I. Responses to tones at the characteristic frequency. J. Neurophysiol. 71: 1022–1036.
Joris PX, Smith PH, Yin TCT (1994b) Enhancement of neural synchronization in the anteroventral cochlear nucleus. II. Responses in the tuning curve tail. J. Neurophysiol. 71: 1037–1051.
Joris PX, Smith PH, Yin TCT (1998) Coincidence detection in the auditory system: Fifty years after Jeffress. Neuron 21: 1235–1238.
Joris PX, Yin TCT (1992) Responses to amplitude-modulated tones in the auditory nerve of the cat. J. Acoust. Soc. Am. 91: 215–232.
Kalluri S (2000) Cochlear nucleus onset neurons studied with mathematical models. Ph.D. thesis, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA.
Kempter R, Gerstner W, van Hemmen JL, Wagner H (1998) Extracting oscillations: Neuronal coincidence detection with noisy periodic spike input. Neural Comput. 10: 1987–2017.
Kenyon GT, Puff RD, Fetz EE (1992) A general diffusion model for analyzing the efficacy of synaptic input to threshold neurons. Biol. Cybern. 67: 133–141.
Kiang NYS (1990) Curious oddments of auditory-nerve studies. Hearing Res. 49: 1–16.
Kiang NYS, Moxon EC (1972) Physiological considerations in arti-ficial stimulation of the inner ear. Ann. Otol. 81: 714–730.
Kiang NYS, Watanabe T, Thomas EC, Clark LF (1965) Discharge Patterns of Single Fibers in the Cat's Auditory Nerve. MIT Press, Cambridge, MA.
Köppl C (1997) Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl. J. Neurosci. 17: 3312–3321.
Kuhlmann L, Burkitt AN, Clark GM (2001) Peak-splitting in the response of the leaky integrate-and-fire neuron model to low frequency periodic input. In: B Lithgow, I Cosic, eds. Proceedings of Biomedical Research in 2001. Victorian Chapter IEEE EMBS, Melbourne, pp. 13–16.
Lavine RA (1971) Phase-locking in the response of single neurons in cochlear nuclear complex of the cat to low-frequency tonal stimuli. J. Neurophysiol. 34: 467–483.
Liberman MC (1982) The cochlear frequency map for the cat: Labeling auditory-nerve fibers of known characteristic frequency. J. Acoust. Soc. Am. 72: 1441–1449.
Liberman MC (1991) Central projections of auditory-nerve fibers of differing spontaneous rate. I. Anteroventral cochlear nucleus. J. Comp. Neurol. 313: 240–258.
Loizou PC (1999) Introduction to cochlear implants. IEEE Eng. Med. Bio. Mag. 18: 32–42.
Lorente de Nó R (1981) The Primary Acoustic Nuclei. Raven Press, New York.
Manis PB, Marx SO (1991) Outward currents in isolated ventral cochlear nucleus neurons. J. Neurosci. 11: 2865–2880.
Marš álek P, Koch C, Maunsell J (1997) On the relationship between synaptic input and spike output jitter in individual neurons. Proc. Natl. Acad. Sci. USA 94: 735–740.
May BJ, Sachs MB (1992) Dynamic range in neural rate responses in the ventral cochlear nucleus of awake cats. J. Neurophys. 68: 1589–1602.
Meddis R, Hewitt MJ, Shackleton TM (1990) Implementation details of a computation model of the inner-hair cell/auditory-nerve synapse. J. Acoust. Soc. Am. 87: 1813–1816.
Mills AW (1958) On the minimum audible angle. J. Acoust. Soc. Am. 30: 237–246.
Molnar CE, Pfeiffer RR (1968) Interpretation of spontaneous spike discharge patterns of neurons in the cochlear nucleus. Proc. IEEE 56: 993–1004.
Neely ST, Kim DO (1986) A model for active elements in cochlear biomechanics. J. Acoust. Soc. Am. 79: 1472–1480.
Oertel D (1983) Synaptic response and electrical properties of cells in brain slices of the mouse anteroventral cochlear nucleus. J. Neurosci. 3: 2043–2053.
Oertel D (1999) The role of timing in the brain stem nuclei of vertebrates. Ann. Rev. Physiol. 61: 497–519.
Osen KK (1969) The intrinsic organization of the cochlear nuclei in the cat. Acta Otolaryngol. 67: 352–359.
Osen KK (1970) Course and terminations of the primary afferents in the cochlear nuclei of the cat: An experimental study. Arch. Ital. Biol. 108: 21–51.
Ostapoff EM, Morest DK (1991) Synaptic organization of globular bushy cells in the ventral cochlear nucleus of the cat: A quantitative study. J. Comp. Neurol. 314: 598–613.
Palmer AR, Russell IJ (1986) Phase-locking in the cochlear-nerve of the guinea-pig and its relation to the receptor potential of inner hair cells. Hearing Res. 24: 1–15.
Paolini AG, Clark GM (1998) Intracellular responses of the rat anteroventral cochlear nucleus to intracochlear electrical stimulation. Brain Res. Bull. 46: 317–327.
Paolini AG, Clark GM, Burkitt AN (1997) Intracellular responses of rat cochlear nucleus to sound and its role in temporal coding. NeuroReport 8: 3415–3422.
Paolini AG, Cotterill EL, Bairaktaris D, Clark GM (1998) Muscimol suppression of the dorsal cochlear nucleus modifies frequency tuning in rats. Brain Res. 785: 309–316.
Paolini AG, FitzGerald JV, Burkitt AN, Clark GM (2001) Temporal processing from the auditory nerve to the medial nucleus of the trapezoid body in the rat.
Papoulis A (1991) Probability, Random Variables, and Stochastic Processes (3rd ed.), McGraw-Hill, Singapore.
Perkel DH, Gerstein GL, Moore GP (1967) Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys. J. 7: 391–418.
Pickles JO (1982) An Introduction to the Physiology of Hearing. Academic Press, London.
Plesser HE, Geisel T (1999) Markov analysis of stochastic resonance in a periodically driven integrate-fire neuron. Phys. Rev. E 59: 7008–7017.
Plesser HE, Tanaka S (1997) Stochastic resonance in a model neuron with reset. Phys. Lett. A 225: 228–234.
Rayleigh Lord (JW Strutt) (1876) On our perception of the direction of a source of sound. Nature Lond. 14: 32–33.
Retzius G (1884) Das Gehöorgan der Wirbeltiere. II. Das Gehörorgant der Reptilien, der Vogel und der Saugetiere. Samson and Wallin, Stockholm.
Rhode WS, Smith PH (1986) Encoding timing and intensity in the ventral cochlear nucleus of the cat. J. Neurophysiol. 56: 261–286.
Rose JE, Brugge JF, Anderson DJ, Hind JE (1967) Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J. Neurophysiol. 30: 769–793.
Rothman JS, Young ED (1996) Enhancement of neural synchronization in computational models of ventral cochlear nucleus bushy cells. Aud. Neurosci. 2: 47–62.
Rothman JS, Young ED, Manis PB (1993) Convergence of auditory nerve fibers onto bushy cells in the ventral cochlear nucleus: Implications of a computational model. J. Neurophysiol. 70: 2562–2583.
Rouiller EM, Cronin-Schreiber R, Fekete DM, Ryugo DK (1986) The central projections of intracellularly labeled auditory nerve fibers in cats: An analysis of terminal morphology. J. Comp. Neurol. 249: 261–278.
Ruggero MA, Rich NC (1987) Timing of spike initiation in cochlear afferents: Dependence on site of innervation. J. Neurophysiol. 58: 379–403.
Ruggero MA, Rich NC, Shivapuja BG, Temchin AN (1995) Auditory-nerve responses to low-frequency tones: Intensity dependence. Aud. Neurosci. 2: 159–186.
Ruggero MA, Robles L, Rich NC (1986) Cochlear microphonics and the initiation of spikes in the auditory nerve: Correlation of single-unit data with neural and receptor potentials recorded from the round window. J. Acoust. Soc. Am. 79: 1491–1498.
Ryugo DK, Fekete DM (1982) Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: A study of the endbulbs of Held. J. Comp. Neurol. 210: 239–257.
Saint-Marie RL, Morest DK, Brandon CJ (1989) The form and distribution of GABAergic synapses on the principal cell types of the ventral cochlear nucleus of the cat. Hearing Res. 42: 97–112.
Salinas E, Sejnowski TJ (2000) Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. J. Neurosci. 20: 6193–6209.
Sento S, Ryugo DK (1989) Endbulbs of Held and spherical bushy cells in cats: Morphological correlates with physiological properties. J. Comp. Neurol. 280: 553–562.
Smith PH, Rhode WS (1987) Characterization of HRP-labeled globular bushy cells in the cat anteroventral cochlear nucleus. J. Comp. Neurol. 266: 360–375.
Tasaki I (1954) Nerve impulses in individual nerve fibres of guinea pig. J. Neurophysiol. 17: 97–122.
Tolbert LP, Morest DK (1982) The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: Electron microscopy. Neurosci. 7: 3053–3068.
Tuckwell HC (1988) Introduction to Theoretical Neurobiology: Nonlinear and Stochastic Theories (Vol. 2). Cambridge University Press, Cambridge.
Tuckwell HC, Richter W (1978) Neuronal interspike time distributions and the estimation of neurophysiological and neuroanatomical paramaters. J. Theor. Biol. 71: 167–183.
von Békésy G (1960) Experiments in Hearing. EG Wever (ed. and trans.). McGraw-Hill, New York.
Walmsley B, Alvarez FJ, Fyffe REW (1998) Diversity of structure and function at mammalian central synapses. Trends Neurosci. 21: 81–88.
Webster DB, Popper AN, Fay RR (eds.) (1992) The Mammalian Auditory Pathway: Neuroanatomy. Springer, New York.
Young ED, Shofner WP, White JA, Robert JM, Voigt HF (1988) Response properties of cochlear nucleus neurons in relationship to physiological mechanisms. In: S Hassler, ed. Functions of the Auditory System. Wiley, New York. pp. 277–312.
Young ED, Rothman JS, Manis PB (1993) Regularity of discharge constrains models of ventral cochlear nucleus bushy cells. In: MA Merchan, J Juiz, eds. The Mammalian Cochlear Nuclei: Organization and Function. Plenum Press, New York. pp. 395–410.
Zhang S, Trussell LO (1994) A characterization of excitatory postsynaptic potentials in the avian nucleus magnocellularis. J. Neurophysiol. 72: 705–718.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuhlmann, L., Burkitt, A.N., Paolini, A. et al. Summation of Spatiotemporal Input Patterns in Leaky Integrate-and-Fire Neurons: Application to Neurons in the Cochlear Nucleus Receiving Converging Auditory Nerve Fiber Input. J Comput Neurosci 12, 55–73 (2002). https://doi.org/10.1023/A:1014994113776
Issue Date:
DOI: https://doi.org/10.1023/A:1014994113776