Abstract
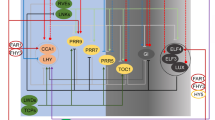
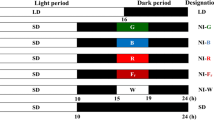
Light strongly influences plant processes and is instrumental inestablishing patterns in photosynthetic responses, enzymatic activity, andlevels of some plant hormones. At this time, it is unclear how the biosynthesisof the plant hormone ethylene is influenced by light in cotton cotyledonarytissue. To answer this question, the cotton (Gossypiumhirsutum L.) cultivar ‘DPL50’ was exposed to thefollowing light and/or dark treatments over a 72-h period: a12-h photoperiod, continuous light, or continuous dark. Ethylene,1-aminocyclopropane-1-carboxylic acid (ACC), andN-malonyl-1-aminocyclopropane-1-carboxylic acid (MACC) were assayed from wholeplant samples. Cotton plants exhibited a pattern of ethylene evolution thatappears to be controlled by a circadian clock. This circadian pattern wassuggested by the lack of change in ethylene evolution rate under continuouslight. The pattern of ethylene evolution was disrupted during a continuous darktreatment, indicating that light in some way is responsible for setting thecircadian clock for ethylene evolution and that light-sensing molecules such asphytochrome may be involved. Patterns of ACC and MACC concentration were notcircadian.
Similar content being viewed by others
References
Amerheun N., Breuning F., Eberle J., Skorupka H. and Tophof S. 1982. The metabolism of 1-aminocyclopropane-1-carboxylic acid. In: Waring P.F. (ed.), Plant Growth Substances. Academic Press, London, pp. 249–258.
Bouzayen M., Latche A., Albert G. and Pech J.-C. 1988. Intercellular sites of synthesis and storage of 1-(malonylamino)cyclopropane-1-carboxylic acid in Acer pseudoplatanus cells. Plant Physiol. 88: 613–617.
Carpenter C.D., Kreps J.A. and Simon A.E. 1994. Genes encoding glycine-rich Arabidopsis thaliana proteins with RNA-binding motifs are influenced by cold treatment and endogenous circadian rhythms. Plant Physiol. 104: 1015–1025.
Cumming B.G. 1969. Circadian rhythms of flower induction and their significance in photoperiodic response. Can. J. Bot. 47: 309–324.
Deikman J. and Hammer P.E. 1995. Induction of anthocyanin accumulation by cytokinin in Arabidopsis thaliana. Plant Physiol. 108: 47–57.
Finlayson S.A., Lee I.-J. and Morgan P.W. 1998. Phytochrome B in the regulation of circadian ethylene production in sorghum. Plant Physiol. 116: 17–25.
Finlayson S.A., Lee I.J., Mullet J.E. and Morgan P.W. 1999. The mechanism of rhythmic ethylene production in sorghum. The role of phytochrome B and simulated shading, Plant Physiol. 119: 1083–1089.
Finlayson S.A. and Morgan P.W. 2000. Shade avoidance and phytochrome B regulation of phytochrome A. In:, American Society of Plant Physiologists Annual Meeting, Abstract #550, San Diego California, July 15-19, 2000.
Fuhrer J. and Fuhrer-Fries C.B. 1985. Formation and transport of 1-aminocyclopropane-1-carboxylic acid in pea plants. Phytochemistry 24: 19–22.
Goeschl J.D., Pratt H.K. and Bonner B.A. 1967. An effect of light on the production of ethylene and the growth of plumular portion of etiolated pea seedlings. Plant Physiol. 42: 1077–1080.
Gorton H.L., Williams W.E. and Assman S.M. 1993. Circadian rhythms in stomatal responsiveness to red and blue light. Plant Physiol. 103: 399–406.
Heintzen C., Fisher R., Melzer S., Kappeler S., Apel K. and Staiger D. 1994a. Circadian oscillations of a transcript encoding a germin-like protein that is associated with cell walls in young leaves of the long-day plant Sinapis alba L. Plant Physiol. 106: 905–915.
Heintzen C., Fisher R., Melzer S., Kappeler S., Apel K. and Staiger D. 1994b. A light-and temperature-entrained circadian clock controls expression of transcripts encoding nuclear proteins with homology to RNA-binding proteins in meristematic tissue. Plant J. 5: 799–813.
Hewett E.W. and Wareing P.F. 1973. Cytokinins in Populus × rubusta (Schneid): light effects on ethylene levels. Planta. 114: 119–129.
Hoffman N.E., Liu Y. and Yang S.F. 1983. Changes in 1-(malonylamino) cyclopropane-1-carboxylic acid content in wilted wheat leaves in relation to their ethylene production rates and 1-aminocyclopropane-1-carboxylic acid content. Planta. 157: 518–523.
Ievinsh G. and Kreicbergs O. 1992. Endogenous rhythmicity of ethylene production in growing intact cereal seedlings. Plant Physiol. 100: 1389–1391.
Imaseki H., Pjon C.J. and Furuya M. 1971. Phytochrome action in Oryza sativa L. IV. Red and far-red reversible effect on the production of ethylene in excised coleoptiles, Plant Physiol. 48: 241–244.
Jiao X.-Z., Philosoph-Hada S., Su L.-Y. and Yang S.F. 1986. The conversion of 1-(malonyl)cyclopropane-1-carboxylic acid to 1-aminocyclopropane-1-carboxylic acid in plant tissue. Plant Physiol. 81: 637–641.
Kannangara T., Durley R.C. and Simpson G.M. 1982. Diurnal changes of leaf water potential, abscisic acid, phaseic acid and indole-3-acetic acid in field grown Sorghum bicolor L. Moench. Z. Pflanzenphysiol. 106: 55–61.
Kapuya J.A. and Hall M.A. 1977. Diurnal variations in endogenous ethylene levels in plants. New Phytol. 79: 233–237.
Kathiresan A., Reid D.M. and Chinnappa C.C. 1996. Light and temperature entrained circadian regulation of activity and mRNA accumulation of 1-aminocyclopropane-1-carboxylic acid oxidase in Stellaria longipes. Planta. 199: 329–335.
Knee M. 1985. Metabolism of 1-aminocyclopropane-1-carboxylic acid during fruit development. J. Exp. Bot. 36: 670–678.
Lipe J.A. and Morgan P.W. 1973. Ethylene, a regulator of young fruit abscission. Plant Physiol. 51: 949–953.
Liu Y., Su L.Y. and Yang S.F. 1985. Ethylene promotes the capability to malonate 1-aminocyclopropane-1-carboxylic acid and D-amino acids in preclimateric tomato fruit. Plant Physiol. 77: 891–895.
Lizada M.C.C. and Yang S.F. 1979. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 100: 140–145.
Machackova I., Chauvaux N., Dewitte W. and van Onckelen H. 1997. Diurnal fluctuation in ethylene formation in Chenopodium rubrum. Plant Physiol. 113: 981–985.
O'Neill S.D., Zhang X.S. and Zheng C.C. 1994. Dark and circadian regulation of mRNA accumulation in the short-day plant Pharbitis nil. Plant Physiol. 104: 569–580.
Pilgrim M.L., Caspar T., Quail P.H. and McClung C.R. 1993. Circadian and light-regulated expression of nitrate reductase in Arabidopsis. Plant Mol. Biol. 23: 349–364.
Rikin A., Chalutz E. and Anderson J.D. 1984. Rhythmicity in ethylene production in cotton seedlings. Plant Physiol. 75: 493–495.
Satoh S. and Esashi Y. 1984. Changes in ethylene production and in 1-aminocyclopropane-1-carboxylic acid (ACC) and malonyl-ACC contents of cocklebur seeds during their development. Plant Cell Physiol. 25: 1277–1283.
Van Loon L.C. and Fontaine J.J.H. 1984. Accumulation of 1-(malonylamino) cyclopropane1-1carboxylic acid in ethylene-synthesizing tobacco leaves. Plant Growth Regul. 2: 227–234.
Watillon B., Kettmann R., Boxus P. and Burny A. 1993. Developmental and circadian pattern of rubisco activase mRNA accumulation in apple plants. Plant Mol. Biol. 23: 501–509.
Yang C., Wessler A. and Wild A. 1993. Studies on the diurnal courses of the contents of abscisic acid, 1-aminocyclopropane-1-carboxylic acid, and its malonyl conjugate in needles of damaged and undamaged spruce trees. J. Plant Physiol. 141: 624–626.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jasoni, R.L., Cothren, J.T., Morgan, P.W. et al. Circadian ethylene production in cotton. Plant Growth Regulation 36, 127–133 (2002). https://doi.org/10.1023/A:1015073400206
Issue Date:
DOI: https://doi.org/10.1023/A:1015073400206