Abstract
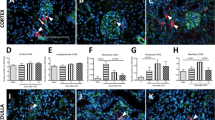
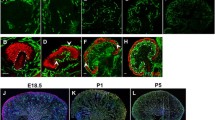
The adult kidney is highly vascular and receives about 20% of the cardiac output, yet the mode of development of the glomerular capillaries is not fully understood. At the inception of nephrogenesis the condensed metanephric mesenchyme contains no patent capillaries. However, in this current study we detected vascular endothelial growth factor (VEGF) mRNA and protein in uninduced mouse E11 metanephric mesenchyme and in cell lines from this tissue. Moreover, transcripts for receptor tyrosine kinases which are markers of endothelial precursors (VEGFR-1/Flt-1, VEGFR-2/Flk-1 and Tie-1) were expressed by the E11 mesenchyme. In transgenic mice, Tie1/LacZ-expressing cells were identified in E11 renal mesenchyme when patent vessels were absent. Moreover, a similar pattern of transgene expression was detected within intermediate mesoderm condensing to form metanephric mesenchyme. When Tie-1/LacZ E11 metanephroi were transplanted into the nephrogenic cortex of wild-type mice, transgene-expressing capillary loops were detected in glomeruli developing in donor tissue. In contrast, glomerular Tie-1/LacZ-positive vessels never developed in rudiments in organ culture. We postulate that endothelial precursors are present at the inception of the mouse nephrogenesis, and these differentiate and undergo morphogenesis into glomerular capillaries when experimental conditions resemble those found in the metanephros in vivo.
Similar content being viewed by others
References
Noden DW. Embryonic origins and assembly of blood vessels. Am Rev Resp Dis 1989; 140, 1097–1103.
Poole TJ, Coffin JD. Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J Exp Zool 1989; 251, 224–231.
Wilting JR, Christ B. Embryonic angiogenesis: a review. Naturwissenschaften 1996; 83, 153–164.
Coffin JD, Poole TJ. Embryonic vascular development: immunohistochemical identification of the origin and subsequent morphogenesis of the major vessel primordia in quail embryos. Development 1988; 102, 735–748.
Pardanaud L, Yassine F, Dieterlen-Lievre F. Relationship between vasculogenesis, angiogenesis and haematopoiesis during avian ontogeny. Development 1989; 105, 473–485.
Coffin JD, Harrison J, Schwartz S, Heimark R. Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Dev Biol 1991; 148, 51–62.
Pardanaud L, Luton D, Prigent M, Bourcheix L-M, Catala M, Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development 1996; 122, 1363–1371.
Mustonen T and Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol 1995; 129, 895–898.
Ferrara N, Bunting S. Vascular endothelial growth factor, a specific regulator of angiogenesis. Curr Opin Nephrol Hyperten 1996; 5, 35–44.
Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 1993; 118, 489–498.
Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests paracrine regulation of murine vascular development. Dev Dynam 1995; 204, 228–239.
Shalaby F, Rossant J, Yamaguchi TP, Gerstenstein M, Wu X-F, Breitman ML and Schuh AC. Failure of blood island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995; 376, 62–66.
Fong G-H, Rossant J, Gerstenstein M, Breitman ML. Role of the flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995; 376, 66–74.
De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelail growth factor. Science 1992; 255, 989–991.
Millauer B, Wizigmann-Voos S, Schnurch H, et al. High affinity VEGF binding and developmental expression suggest flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993; 72, 835–846.
Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380, 435–439.
Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380, 439–442.
Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. tek, a novel tyrosine kinase gene located on chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene 1992; 7, 1471–1480.
Sato TN, Qin Y, Kozak CA, Andus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci USA 1993; 90, 9355–9358.
Korhonen J, Polvi A, Partanen J, Alitalo K. The mouse tie receptor tyrosine kinase gene: expression during embryonic angiogenesis. Oncogene 1994; 9, 395–403.
Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J 1995; 14, 5884–5891.
Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995; 376, 70–74.
Rodewald HR, Sato TN. Tie 1, a receptor tyrosine kinase essential for vascular endothelial cell integrity, is not critical for the development of hematopoietic cells. Oncogene 1996; 12, 397–404.
Partanen J, Puri MC, Schwartz L, Fischer KD, Bernstein A, Rossant J. Cell autonomous functions of the receptor tyrosine kinase TIE in late phases of angiogenic capillary growth and endothelial cell survival during murine development. Development 1996; 122, 3013–3021.
Dumont DJ, Gradwohl G, Fong, G-H, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis in the embryo. Genes Dev 1994; 8, 1897–1909.
Suri C, Jones PF, Patan S, et al. Requisite role of Angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996; 87, 1171–1180.
Bernstein J, Cheng F, Roska J. Glomerular differentiation in metanephric culture. Lab Invest 1981; 45, 183–190.
Sariola H, Ekblom P, Lehtonen E, Saxen L. Differentiation and vascularisation of the metanephric kidney grafted on the chorioallantoic membrane. Dev Biol 1983; 96, 427–435.
Sariola H, Timpl R, von der Mark K, et al. Dual origin of glomerular basement membrane. Dev Biol 1984; 101, 86–96.
Loughna S, Landels EC, Woolf AS. Growth factor control of developing kidney endothelial cells. Exp Nephrol 1996; 4, 112–118.
Grobstein C. Inductive interaction in the development of the mouse metanephros. J Exp Zool 1955; 130, 319–340.
Hyink DP, Tucker DC, St John PL, et al. Endogenous origin of glomerular endothelial and mesangial cells in grafts of embryonic kidneys. Am J Physiol 1996; 270, F886-F899.
Robert B, St John PL, Hyink DP, Abrahamson DR. Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol 1996; 271, F744-F753.
Korhonen J, Lahtinen I, Halmekyto M, et al. Endothelial-specific gene expression directed by the tie gene promoter in vivo. Blood 1995; 86, 1828–1835.
Woolf AS, Kolatsi-Joannou M, Hardman P, et al. Roles of hepatocyte growth factor/scatter factor and met in early development of the metanephros. J Cell Biol 1995; 128, 171–184.
Woolf AS, Bosch RJ, Fine LG. Gene transfer into the mammalian kidney: microtransplantation of retrovirus-transduced metanephric tissue. Exp Nephrol 1993; 1, 41–48.
Kolatsi-Joannou M, Woolf AS, Hardman P, Gordge M, White SJ, Henderson R. The hepatocyte growth factor/scatter factor (HGF/SF) receptor, met, transduces a morphogenetic signal in renal glomerular fibromuscular mesangial cells. J Cell Sci 1995; 108, 3703–3714.
Woolf AS, Palmer SJ, Snow ML, Fine LG. Creation of a functioning chimeric mammalian kidney. Kidney Int 1990; 38, 991–997.
Hamburger V. A Manual of Experimental Embryology 5th impression. Chicago, IL: University of Chicago Press 1973: 158–163.
Oelrichs R, Reid HH, Bernard O, Ziemiecki A, Wilks A. NYK/FLK-1: a putative receptor tyrosine kinase isolated from E10 embryonic neuroepithelium is expressed in endothelial cells of the developing embryo. Oncogene 1993; 8, 11–18.
Dumont DJ, Fong G-H, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularisation of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dynam 1995; 203, 80–92.
Fong G-H, Klingensmith J, Wood CR, Rossant J, Breitmen ML. Regulation of flt-1 expression during mouse embryogenesis suggests a role in the establishment of vascular endothelium. Dev Dynam 1996; 207, 1–10.
Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992; 114, 521–532.
Woolf AS, Loughna S. Origin of glomerular capillaries: is the verdict in? Exp Nephrol 1997; in press.
O'Reilly MS, Holmgren L, Shing, Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinome. Cell 1994; 79, 315–328.
Tufro-McReddie A, Norwood VF, Aylor KW, Botkin SJ, Carey RM, Gomez RA. Oxygen regulates vascular endothelial growth factor-mediated vasculogenesis and tubulogenesis. Dev Biol 1997; 183, 139–149.
Finnerty H, Kelleher K, Morris GE, et al. Molecular cloning of murine FLT and FLT4. Oncogene 1993; 8, 2293–2298.
Matthews W, Jordan CT, Gavin M, Jenkins NA, Copeland NG, Lemischka IR. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci USA 1991; 88, 9026–9030.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Loughna, S., Hardman, P., Landels, E. et al. A molecular and genetic analysis of renalglomerular capillary development. Angiogenesis 1, 84–101 (1997). https://doi.org/10.1023/A:1018357116559
Issue Date:
DOI: https://doi.org/10.1023/A:1018357116559