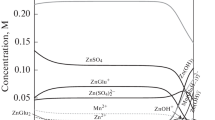
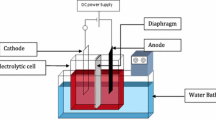
Abstract
The effects of 4-ethylpyridine and 2-cyanopyridine on the electrowinning of zinc in the presence and absence of antimony have been studied. The results are compared with those of a common industrial additive, gum arabic. Addition of either compound reduced current efficiency, increased power consumption and lowered the surface quality of electrodeposited zinc. Both the additives showed similar polarization behaviour to gum arabic. Addition of 0.04mgdm−3 antimony increased current efficiency, reduced power consumption and altered the surface morphology and crystallographic orientations. Combinations of antimony with 4-ethylpyridine resulted in very good current efficiencies, and zinc morphology and quality.
Similar content being viewed by others
References
F. Mansfeld and S. Gilman, J. Electrochem. Soc., 117 (1970) 1521.
Idem, ibid. 117 (1970) 1150.
Idem, ibid. 117 (1970) 588.
J. W. Diggle and A. Damjanovic, ibid. 119 (1972) 1649.
V. V. Romanov, Sov. Electrochem. 7 (1971) 1400.
S. Higuchi and Y. Miyake, Denkikagaku Electrochem. 39 (1971) 896.
S. Higuchi, S. Takahashi and Y. Miyake, ibid. 39 (1971) 522.
I. N. Justinijanovic, J. N. Jovicevic and A. R. Despic, J. Appl. Electrochem. 3 (1973) 193.
J. Bressan and R. Wiart, J. Appl. Electrochem. 7 (1977) 505.
Idem, ibid. 9(1979) 43.
D. L. Piron, D. Mathieu and M. D'Amboise, Canad. J. Chem. Eng. 65 (1981) 685.
D. J. MacKinnon, J. M. Brannen and R. M. Morrison, J. Appl. Electrochem. 18 (1988) 252.
D. J. MacKinnon, and J. M. Brannen, ibid. 12 (1982) 21.
D. J. MacKinnon, J. M. Brannen and R. M. Morrison, ibid. 12 (1982) 39.
B. K. Thomas and D. J. Fray, ibid. 11 (1981) 677.
A. Hosny, Hydrometallurgy, 32 (1993) 261.
M. Karavastev and St. Karaivanov, ibid. 23 (1993) 763.
M. Karavastev, Hydrometallurgy, 35 (1994) 391.
R. S. Dubey, S. N. Upadhyay and J. S. Choudhary, J. Electrochem. Soc. India 42 (1993) 239.
Kirk-Othmer, `Encyclopedia of Chemical Technology', Vol. 7, `Corrosion and Corrosion Inhibitors', Wiley-Interscience, New York, (1982), p. 135.
P. N. S. Yadav and R. Wadhawani, Trans. SAEST 28, (1993) 134.
H. C. Brown, Trans. Inst. Met. Finish. 43 (1969) 63.
W. Gundel and W. Strauss, US Patent, 2876 177 (1959).
N. Hackerman and H. Haeshe, J. Electrochem. Soc. 105 (1958) 191.
D. J. MacKinnon, R. M. Morrison, J. E. Mouland and P. E. Warren, J. Appl. Electrochem. 20 (1990) 728.
D. J. Robinson and T. J. O'Keefe, ibid. 6 (1976) 1.
S. E. Afifi, A. R. Ebaid, M. M. Hegazy and A. K. Barakat, J. Metals 44 (1992) 32.
S. C. Das, P. Singh and G. Hefter, J. Appl. Electrochem. in press.
C. L. Mantell, `Electrochemical Engineering', 4th edition. McGraw-Hill, New York, (1960), p. 210.
G. T. Wever, J. Metals 11 (1959) 130.
D. J. MacKinnon and J. M. Brannen, J. Appl. Electrochem. 7(1977) 451.
P. A. Adcock, A. R. Ault and O. M. G. Newman, ibid. 15 (1985) 865.
C. Cachet and R. Wiart. ibid. 20 (1990) 1009.
U. F. Turomshina and V. V. Stender, J. Appl. Chem., USSR 28 (1955) 151, 347, 447.
P. Moore and R. G. Wilkins, J. Chem. Soc. (1964) 3455.
Ya. I. Turiyan, N. I. Pershakova and O. E. Ruvinskii, Zh. Obschei. Khim. 42 (1972) 1198.
Corwin Hansch and Albert Leo, `Exploring QSAR: Fun-damentals and Applications in Chemistry and Biology', American Chemical Society (1995).
G. T. Hefter and M. Salomon, J. Soln Chem. 23 (1994) 579.
D. R. Fosnacht and T. J. O'Keefe, Met. Trans. 14B (1983) 645.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DAS , S.C., SINGH , P. & HEFTER , G.T. The effects of 4-ethylpyridine and 2-cyanopyridine on zinc electrowinning from acidic sulfate solutions. Journal of Applied Electrochemistry 27, 738–744 (1997). https://doi.org/10.1023/A:1018452224138
Issue Date:
DOI: https://doi.org/10.1023/A:1018452224138