Abstract
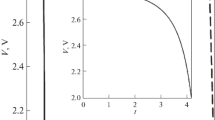
Simplified models based on porous electrode theory are used to describe the discharge of rechargeable lithium batteries and derive analytic expressions for the specific capacity against discharge rate in terms of the relevant system parameters. The resulting theoretical expressions are useful for design and optimization purposes and can also be used as a tool for the identification of system limitations from experimental data. Three major cases are considered that are expected to hold for different classes of systems being developed in the lithium battery industry. The first example is a cell with solution phase diffusion limitations for the two extreme cases of a uniform and a completely nonuniform reaction rate distribution in the porous electrode. Next, a discharge dominated by solid phase diffusion limitations inside the insertion electrode particles is analysed. Last, we consider an ohmically-limited cell with no concentration gradients and having an insertion reaction whose open-circuit potential depends linearly on state of charge. The results are applied to a cell of the form Li|solid polymer electrolyte|LiyMn2O4 in order to illustrate their utility.
Similar content being viewed by others
References
R. Selim and P. Bro, J. Electrochem. Soc. 118 (1971) 829.
D. Linden (ed.),`Handbook of Batteries', 2nd edn, McGraw-Hill, New York (1995).
G. W. Vinal, `Storage Batteries', 4th edn, J. Wiley & Sons, New York (1995), p.216.
M. Z. A. Munshi and B. B. Owens, Solid State lon. 38 (1990) 103.
M. Doyle, T. F. Fuller and J. Newman, J. Electrochem. Soc. 140 (1993) 1526.
T. F. Fuller, M. Doyle and J. Newman, ibid. 141 (1994) 1.
Idem, ibid. 141 (1994) 982.
M. Doyle, T. F. Fuller and J. Newman, Electrochim. Acta 39 (1994) 2073.
M. Doyle, J. Newman, A. S. Gozdz, C. N. Schmutz and J.-M. Tarascon, J. Electrochem. Soc. 143 (1996) 1890.
W. Tiedemann and J. Newman, ibid. 122 (1975) 1482.
J. Newman, ibid. 142 (1995) 97.
J. Newman and C. W. Tobias, ibid. 109 (1962) 1183.
R. Pollard and J. Newman, Electrochim. Acta 25 (1980) 315.
W. Stein, Naturwissenschaften 45 (1958) 459.
M. Doyle and J. Newman, J. Power Sources 54 (1995) 46.
S. Atlung, B. Zachau-Christiansen, K. West and T. Jacobsen, J. Electrochem. Soc. 131 (1984) 1200.
B. C. Knutz, K. West, B. Zachau-Christiansen and S. Atlung, J. Power Sources 43-44 (1993) 733.
J. Newman, `Electrochemical Systems', Prentice Hall, Englewood Cliffs, N. J. (1991).
D. A. G. Bruggeman, Ann. Phys. 24 (1935) 636.
H. S. Carslaw and J. C. Jaeger, `Conduction of Heat in Solids', Clarendon Press, Oxford (1959), p. 242.
F. M. Gray, `Solid Polymer Electrolytes', VCH, New York (1991).
A. Bouridah, F. Dalard, D. Deroo and M. B. Armand, J. Appl. Electrochem. 17 (1987) 625.
D. Guyomard and J. M. Tarascon, J. Electrochem. Soc. 139 (1992) 937.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DOYLE , M., NEWMAN , J. Analysis of capacity–rate data for lithium batteries using simplified models of the discharge process. Journal of Applied Electrochemistry 27, 846–856 (1997). https://doi.org/10.1023/A:1018481030499
Issue Date:
DOI: https://doi.org/10.1023/A:1018481030499