Abstract
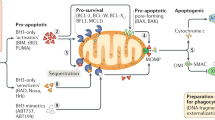
The Bcl-2 family of proteins are important regulators of cell death. They are comprised of two opposing factions, the proapoptotic versus the antiapoptotic members. Both are required for normal development and cellular homeostasis of the immune system and other tissues. However, in certain circumstances they may participate in the development of disease.
Similar content being viewed by others
REFERENCES
Kerr JF, Wyllie AH, Currie AR: Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257, 1972
Raff MC: Social controls on cell survival and cell death. Nature 356:397–400, 1992
Vaux DL, Korsmeyer SJ: Cell death in development. Cell 96:245–254, 1999
Vaux DL, Haecker G, Strasser A: An evolutionary perspective on apoptosis. Cell 76:777–779, 1994
Barr PJ, Tomei LD: Apoptosis and its role in human disease. Biotechnology (NY) 12:487–493, 1994
Thompson CB: Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462, 1995
Strasser A, Huang DC, Vaux DL: The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochim Biophys Acta 1333:F151-F178, 1997
Thornberry NA, Lazebnik Y: Caspases: Enemies within. Science 281:1312–1316, 1998
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S: A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50, 1998
Andrade F, Roy S, Nicholson D, Thornberry N, Rosen A, Casciola-Rosen L: Granzyme B directly and efficiently cleaves several downstream caspase substrates: Implications for CTL-induced apoptosis. Immunity 8:451–460, 1998
Scaffidi C, Kirchhoff S, Krammer PH, Peter ME: Apoptosis signaling in lymphocytes. Curr Opin Immunol 11:277–285, 1999
Ashkenazi A, Dixit VM: Death receptors: Signaling and modulation. Science 281:1305–1308, 1998
Smith CA, Farrah T, Goodwin RG: The TNF receptor superfamily of cellular and viral proteins: Activation, costimulation, and death. Cell 76:959–962, 1994
Nagata S: Apoptosis by death factor. Cell 88:355–365, 1997
Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM: FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505–512, 1995
Hsu H, Xiong J, Goeddel DV: The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 81:495–504, 1995
Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D: A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem 270:7795–7798, 1995
Boldin MP, Goncharov TM, Goltsev YV, Wallach D: Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor-induced cell death. Cell 85:803–815, 1996
Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, et al.: FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817–827, 1996
Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM: An induced proximity model for caspase-8 activation. J Biol Chem 273:2926–2930, 1998
Martin DA, Siegel RM, Zheng L, Lenardo MJ: Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHα1) death signal. J Biol Chem 273:4345–4349, 1998
Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, et al.: Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94:339–352, 1998
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, et al.: Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325–337, 1998
Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, et al.: Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94:739–750, 1998
Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P: Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94:727–737, 1998
Strasser A, Harris AW, Huang DC, Krammer PH, Cory S: Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 14:6136–6147, 1995
Memon SA, Moreno MB, Petrak D, Zacharchuk CM: Bcl-2 blocks glucocorticoid-but not Fas-or activation-induced apoptosis in a T cell hybridoma. J Immunol 155:4644–4652, 1995
Huang DC, Cory S, Strasser A: Bcl-2, Bcl-xL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene 14:405–414, 1997
Wallach D, Kovalenko AV, Varfolomeev EE, Boldin MP: Death-inducing functions of ligands of the tumor necrosis factor family: A Sanhedrin verdict. Curr Opin Immunol 10:279–288, 1998
Tartaglia LA, Goeddel DV: Tumor necrosis factor receptor signaling. A dominant negative mutation suppresses the activation of the 55-kDa tumor necrosis factor receptor. J Biol Chem 267:4304–4307, 1992
Malinin NL, Boldin MP, Kovalenko AV, Wallach D: MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 385:540–544, 1997
Yin Foo S, Nolan GP: NF-κB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet 15:229–235, 1999
Zhang J, Cado D, Chen A, Kabra NH, Winoto A: Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392:296–300, 1998
Newton K, Harris AW, Bath ML, Smith KGC, Strasser A: A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J 17:706–718, 1998
Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, et al.: FADD: Essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279:1954–1958, 1998
Smith KG, Strasser A, Vaux DL: CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO J 15:5167–5176, 1996
Chinnaiyan AM, Orth K, O'Rourke K, Duan H, Poirier GG, Dixit VM: Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem 271:4573–4576, 1996
Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, et al.: Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apol, and DR3 and is lethal prenatally. Immunity 9:267–276, 1998
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T: p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362:847–849, 1993
Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, et al.: Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362:849–852, 1993
Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH: p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following γ-irradiation. Oncogene 9:1767–1773, 1994
Strasser A, Harris AW, Jacks T, Cory S: DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 79:329–339, 1994
Miyashita T, Reed JC: Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299, 1995
McCurrach ME, Connor TM, Knudson CM, Korsmeyer SJ, Lowe SW: bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci USA 94:2345–2349, 1997
Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T: Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385:637–640, 1997
Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, et al.: Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284:156–159, 1999
Miyashita T, Reed JC: bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res 52:5407–5411, 1992
Adams JM, Cory S: The Bcl-2 protein family: Arbiters of cell survival. Science 281:1322–1326, 1998
Tsujimoto Y, Cossman J, Jaffe E, Croce CM: Involvement of the bcl-2 gene in human follicular lymphoma. Science 228:1440–1443, 1985
Vaux DL, Cory S, Adams JM: Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335:440–442, 1988
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, et al.: bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597–608, 1993
Gibson L, Holmgreen SP, Huang DC, Bernard O, Copeland NG, Jenkins NA, et al.: bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene 13:665–675, 1996
Song Q, Kuang Y, Dixit VM, Vincenz C: Boo, a novel negative regulator of cell death, interacts with Apaf-1. EMBO J 18:167–178, 1999
Lin EY, Orlofsky A, Wang HG, Reed JC, Prystowsky MB: A1, a Bcl-2 family member, prolongs cell survival and permits myeloid differentiation. Blood 87:983–992, 1996
Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW: MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to bcl-2. Proc Natl Acad Sci USA 90:3516–3520, 1993
Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ: Bcl-xL and Bcl-2 repress a common pathway of cell death. J Exp Med 182:821–828, 1995
Zhou P, Qian L, Bieszczad CK, Noelle R, Binder M, Levy NB, et al.: Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood 92:3226–3239, 1998
Grillot DA, Merino R, Nunez G: Bcl-XL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med 182:1973–1983, 1995
Strasser A, Harris AW, Cory S: bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67:889–899, 1991
Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ: bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67:879–888, 1991
Nguyen M, Millar DG, Yong VW, Korsmeyer SJ, Shore GC: Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J Biol Chem 268:25265–25268, 1993
Lithgow T, van Driel R, Bertram JF, Strasser A: The protein product of the oncogene bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane. Cell Growth Different 5:411–417, 1994
Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC: Investigation of the subcellular distribution of the Bcl-2 oncoprotein: Residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res 53:4701–4714, 1993
Oltvai ZN, Milliman CL, Korsmeyer SJ: Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619, 1993
Chittenden T, Harrington EA, O'Connor R, Flemington C, Lutz RJ, Evan GI, et al.: Induction of apoptosis by the Bcl-2 homologue Bak. Nature 374:733–736, 1995
Kiefer MC, Brauer MJ, Powers VC, Wu JJ, Umansky SR, Tomei LD, et al.: Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature 374:736–739, 1995
Farrow SN, White JH, Martinou I, Raven T, Pun KT, Grinham CJ, et al.: Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature 374:731–733, 1995
Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJ: Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci USA 94:12401–12406, 1997
Inohara N, Ekhterae D, Garcia I, Carrio R, Merino J, Merry A, et al.: Mtd, a novel Bcl-2 family member activates apoptosis in the absence of heterodimerization with Bcl-2 and Bcl-xL. J Biol Chem 273:8705–8710, 1998
Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ: Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell 80:285–291, 1995
Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, et al.: Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 11:1921–1928, 1995
Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ: BID: A novel BH3 domain-only death agonist. Genes Dev 10:2859–2869, 1996
Inohara N, Ding L, Chen S, Nunez G: Harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L). EMBO J 16:1686–1694, 1997
Imaizumi K, Tsuda M, Imai Y, Wanaka A, Takagi T, Tohyama M: Molecular cloning of a novel polypeptide, DP5, induced during programmed neuronal death. J Biol Chem 272:18842–18848, 1997
Hegde R, Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES: Blk, a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-xL, is a potent death agonist. J Biol Chem 273:7783–7786, 1998
O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, et al.: Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO J 17:384–395, 1998
Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, et al.: A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J 14:5589–5596, 1995
Kelekar A, Thompson CB: Bcl-2-family proteins: The role of the BH3 domain in apoptosis. Trends Cell Biol 8:324–330, 1998
Hsu YT, Youle RJ: Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem 273:10777–10783, 1998
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ: Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139:1281–1292, 1997
Hsu YT, Wolter KG, Youle RJ: Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA 94:3668–3672, 1997
Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A: The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell 3:287–296, 1999
Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ: BH3 domain of BAD is required for heterodimerization with BCL-xL and pro-apoptotic activity. J Biol Chem 272:24101–24104, 1997
Oltvai ZN, Korsmeyer SJ: Checkpoints of dueling dimers foil death wishes. Cell 79:189–192, 1994
Yin XM, Oltvai ZN, Korsmeyer SJ: BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369:321–323, 1994
Hu Y, Benedict MA, Wu D, Inohara N, Nunez G: Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci USA 95:4386–4391, 1998
Pan G, O'Rourke K, Dixit VM: Caspase-9, Bcl-xL, and Apaf-1 form a ternary complex. J Biol Chem 273:5841–5845, 1998
Chinnaiyan AM, O'Rourke K, Lane BR, Dixit VM: Interaction of CED-4 with CED-3 and CED-9: A molecular framework for cell death. Science 275:1122–1126, 1997
Martinou JC: Apoptosis. Key to the mitochondrial gate. Nature 399:411–412, 1999
Green DR, Reed JC: Mitochondria and apoptosis. Science 281:1309–1312, 1998
Spector MS, Desnoyers S, Hoeppner DJ, Hengartner MO: Interaction between the C. elegans cell-death regulators CED-9 and CED-4. Nature 385:653–656, 1997
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490, 1998
Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, et al.: Bcl-xL forms an ion channel in synthetic lipid membranes. Nature 385:353–357, 1997
Shimizu S, Narita M, Tsujimoto Y: Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399:483–487, 1999
Mannella CA: Conformational changes in the mitochondrial channel protein, VDAC, and their functional implications. J Struct Biol 121:207–218, 1998
Colombini M, Blachly-Dyson E, Forte M: Ion Channels. New York, Plenum Press, 1996
Hengartner MO: Apoptosis. Death cycle and Swiss army knives. Nature 391:441–442, 1998
Raff M: Cell suicide for beginners. Nature 396:119–122, 1998
Li H, Zhu H, Xu CJ, Yuan J: Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501, 1998
von Freeden-Jeffry U, Solvason N, Howard M, Murray R: The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity 7:147–154, 1997
Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A: Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1-/-mice. Cell 89:1011–1019, 1997
Lagasse E, Weissman IL: Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell 89:1021–1031, 1997
Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL: Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89:1033–1041, 1997
Iwahashi H, Hanafusa T, Eguchi Y, Nakajima H, Miyagawa J, Itoh N, et al.: Cytokine-induced apoptotic cell death in a mouse pancreatic β-cell line: inhibition by Bcl-2. Diabetologia 39:530–536, 1996
Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ: Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3–3 not Bcl-xL. Cell 87:619–628, 1996
Cory S, Harris AW, Strasser A: Insights from transgenic mice regarding the role of bcl-2 in normal and neoplastic lymphoid cells. Philos Trans R Soc Lond B Biol Sci 345:289–295, 1994
McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, et al.: bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57:79–88, 1989
Yang E, Korsmeyer SJ: Molecular thanatopsis: A discourse on the BCL2 family and cell death. Blood 88:386–401, 1996
Chao DT, Korsmeyer SJ: BCL-2 family: Regulators of cell death. Annu Rev Immunol 16:395–419, 1998
Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, et al.: Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA 95:12424–12431, 1998
Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, et al.: Testicular degeneration in Bclw-deficient mice. Nature Genet 18:251–256, 1998
Michaelidis TM, Sendtner M, Cooper JD, Airaksinen MS, Holtmann B, Meyer M, et al.: Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron 17:75–89, 1996
Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, et al.: Bcl-2 deficiency in mice leads to pleiotropic abnormalities: Accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res 55:354–359, 1995
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ: Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229–240, 1993
Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, et al.: Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506–1510, 1995
Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ: Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270:96–99, 1995
Deckwerth TL, Elliott JL, Knudson CM, Johnson EM Jr, Snider WD, Korsmeyer SJ: BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17:401–411, 1996
Brady HJ, Gil-Gomez G, Kirberg J, Berns AJ: Bax α perturbs T cell development and affects cell cycle entry of T cells. EMBO J 15:6991–7001, 1996
Brady HJ, Salomons GS, Bobeldijk RC, Berns AJ: T cells from baxα transgenic mice show accelerated apoptosis in response to stimuli but do not show restored DNA damage-induced cell death in the absence of p53. gene product in. EMBO J 15:1221–1230, 1996
Shindler KS, Latham CB, Roth KA: Bax deficiency prevents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci 17:3112–3119, 1997
Knudson CM, Korsmeyer SJ: Bcl-2 and Bax function independently to regulate cell death. Nature Genet 16:358–363, 1997
Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, et al.: Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275:967–969, 1997
Strasser A, Harris AW, Bath ML, Cory S: Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348:331–333, 1990
McDonnell TJ, Korsmeyer SJ: Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature 349:254–256, 1991
Strasser A, Harris AW, Cory S: Eμ-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene 8:1–9, 1993
Linette GP, Hess JL, Sentman CL, Korsmeyer SJ: Peripheral T-cell lymphoma in lck pr -bcl-2 transgenic mice. Blood 86:1255–1260, 1995
Strasser A, Elefanty AG, Harris AW, Cory S: Progenitor tumours from Eμ-bcl-2-myc transgenic mice have lymphomyeloid differentiation potential and reveal developmental differences in cell survival. EMBO J 15:3823–3834, 1996
Jager R, Herzer U, Schenkel J, Weiher H: Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene 15:1787–1795, 1997
Shi Y, Glynn JM, Guilbert LJ, Cotter TG, Bissonnette RP, Green DR: Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science 257:212–214, 1992
Sakamuro D, Eviner V, Elliott KJ, Showe L, White E, Prendergast GC: c-myc induces apoptosis in epithelial cells by both p53-dependent and p53-independent mechanisms. Oncogene 11:2411–2418, 1995
Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, et al.: Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119–128, 1992
Tamiya S, Etoh K, Suzushima H, Takatsuki K, Matsuoka M: Mutation of CD95 (Fas/Apo-1) gene in adult T-cell leukemia cells. Blood 91:3935–3942, 1998
Beltinger C, Kurz E, Bohler T, Schrappe M, Ludwig WD, Debatin KM: CD95 (APO-1/Fas) mutations in childhood T-lineage acute lymphoblastic leukemia. Blood 91:3943–3951, 1998
Peng SL, Robert ME, Hayday AC, Craft J: A tumor-suppressor function for Fas (CD95) revealed in T cell-deficient mice. J Exp Med 184:149–154, 1996
Davidson WF, Giese T, Fredrickson TN: Spontaneous development of plasmacytoid tumors in mice with defective Fas-Fas ligand interactions. J Exp Med 187:1825–1838, 1998
Zornig M, Grzeschiczek A, Kowalski MB, Hartmann KU, Moroy T: Loss of Fas/Apo-1 receptor accelerates lymphomagenesis in Eμ L-MYC transgenic mice but not in animals infected with MoMuLV. Oncogene 10:2397–2401, 1995
O'Connell J, Bennett MW, O'Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: cancer as a site of immune privilege. Immunol Today 20:46–52, 1999
Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, et al.: Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science 274:1363–1366, 1996
Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D, et al.: Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells-a mechanism of immune evasion? Nat Med 2:1361–1366, 1996
Fisher DE: Apoptosis in cancer therapy: crossing the threshold. Cell 78:539–542, 1994
Levine AJ: p53, the cellular gatekeeper for growth and division. Cell 88:323–331, 1997
Levine AJ, Momand J, Finlay CA: The p53 tumour suppressor gene. Nature 351:453–456, 1991
Hollstein M, Sidransky D, Vogelstein B, Harris CC: p53 mutations in human cancers. Science 253:49–53, 1991
Lowe SW, Ruley HE, Jacks T, Housman DE: p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957–967, 1993
Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al.: High expression of Bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 81:3091–3096, 1993
Brown JM, Wouters BG: Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res 59:1391–1399, 1999
Nossal GJ: Negative selection of lymphocytes. Cell 76:229–239, 1994
Murphy KM, Heimberger AB, Loh DY: Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250:1720–1723, 1990
Swat W, Ignatowicz L, von Boehmer H, Kisielow P: Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature 351:150–153, 1991
Clayton LK, Ghendler Y, Mizoguchi E, Patch RJ, Ocain TD, Orth K, et al.: T-Cell receptor ligation by peptide/MHC induces activation of a caspase in immature thymocytes: the molecular basis of negative selection. EMBO J 16:2282–2293, 1997
Strasser A, Harris AW, von Boehmer H, Cory S: Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci USA 91:1376–1380, 1994
Izquierdo M, Grandien A, Criado LM, Robles S, Leonardo E, Albar JP, et al.: Blocked negative selection of developing T cells in mice expressing the baculovirus p35 caspase inhibitor. EMBO J 18:156–166, 1999
Villa P, Kaufmann SH, Earnshaw WC: Caspases and caspase inhibitors. Trends Biochem Sci 22:388–393, 1997
Nicholson DW, Thornberry NA: Caspases: Killer proteases. Trends Biochem Sci 22:299–306, 1997
Brunner T, Mueller C: Is autoimmunity coming to a Fas(t) end?. Nat Med 5:19–20, 1999
Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, et al.: Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med 181:71–77, 1995
Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH: Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373:438–441, 1995
Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, et al.: Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373:444–448, 1995
Strasser A: Apoptosis. Death of a T cell. Nature 373:385–386, 1995
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S: Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314–317, 1992
Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al.: Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81:935–946, 1995
Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, et al.: Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268:1347–1349, 1995
Kasahara Y, Wada T, Niida Y, Yachie A, Seki H, Ishida Y. et al.: Novel Fas (CD95/APO-1) mutations in infants with a lymphoproliferative disorder. Int Immunol 10:195–202, 1998
Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, et al.: Clinical, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood 89:1341–1348, 1997
Puck JM, Sneller MC: ALPS: An autoimmune human lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Semin Immunol 9:77–84, 1997
Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB: Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med 335:1643–1649, 1996
Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, et al.: Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA 88:8661–8665, 1991
Zhou T, Song L, Yang P, Wang Z, Lui D, Jope RS: Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T cell-mediated autoimmune diseases. Nat Med 5:42–48, 1999
Strasser A, O'Connor L: Fas ligand—Caught between Scylla and Charybdis. Nat Med 4:21–22, 1998
Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ: Bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development 120:301–311, 1994
Farlie PG, Dringen R, Rees SM, Kannourakis G, Bernard O: bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc Natl Acad Sci USA 92:4397–4401, 1995
Dubois-Dauphin M, Frankowski H, Tsujimoto Y, Huarte J, Martinou JC: Neonatal motoneurons overexpressing the bcl-2 protooncogene in transgenic mice are protected from axotomy-induced cell death. Proc Natl Acad Sci USA 91:3309–3313, 1994
Chen DF, Schneider GE, Martinou JC, Tonegawa S: Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature 385:434–439, 1997
Allsopp TE, Wyatt S, Paterson HF, Davies AM: The protooncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell 73:295–307, 1993
Greenlund LJ, Korsmeyer SJ, Johnson EM Jr: Role of BCL-2 in the survival and function of developing and mature sympathetic neurons. Neuron 15:649–661, 1995
Jenner P, Olanow CW: Understanding cell death in Parkinson's disease. Ann Neurol 44:S72-S84, 1998
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD: Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1:1269, 1989
Olanow CW, Tatton WG: Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci 22:123–144, 1999
Marsden CD, Olanow CW: The causes of Parkinson's disease are being unraveled and rational neuroprotective therapy is close to reality. Ann Neurol 44:S189-S196, 1998
Blomer U, Kafri T, Randolph-Moore L, Verma IM, Gage FH: Bcl-xL protects adult septal cholinergic neurons from axotomized cell death. Proc Natl Acad Sci USA 95:2603–2608, 1998
Barinaga M: Stroke-damaged neurons may commit cellular suicide. Science 281:1302–1303, 1998
MacManus JP, Buchan AM, Hill IE, Rasquinha I, Preston E: Global ischemia can cause DNA fragmentation indicative of apoptosis in rat brain. Neurosci Lett 164:89–92, 1993
Nicholls D, Attwell D: The release and uptake of excitatory amino acids. Trends Pharmacol Sci 11:462–468, 1990
Choi DW: Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11:465–469, 1988
Choi DW: Ischemia-induced neuronal apoptosis. Curr Opin Neurobiol 6:667–672, 1996
Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, et al.: Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 13:1017–1030, 1994
Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, et al.: Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke 29:2616–2621, 1998
Lee JM, Zipfel GJ, Choi DW: The changing landscape of ischaemic brain injury mechanisms. Nature 399:A7-A14, 1999
Ma J, Endres M, Moskowitz MA: Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischaemia in mice. Br J Pharmacol 124:756–762, 1998
Forloni G, Bugiani O, Tagliavini F, Salmona M: Apoptosis-mediated neurotoxicity induced by β-amyloid and PrP fragments. Mol Chem Neuropathol 28:163–171, 1996
Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, et al.: Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-β precursor protein and amyloidogenic A β peptide formation. Cell 97:395–406, 1999
Barinaga M: Is apoptosis key in Alzheimer's disease? Science 281:1303–1304, 1998
Wolozin B, Iwasaki K, Vito P, Ganjei JK, Lacana E, Sunderland T, et al.: Participation of presenilin 2 in apoptosis: Enhanced basal activity conferred by an Alzheimer mutation. Science 274:1710–1713, 1996
Selkoe DJ: Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399:A23-A31, 1999
Everett H, McFadden G: Apoptosis: An innate immune response to virus infection. Trends Microbiol 7:160–165, 1999
Meinl E, Fickenscher H, Thome M, Tschopp J, Fleckenstein B: Anti-apoptotic strategies of lymphotropic viruses. Immunol Today 19:474–479, 1998
Barry M, McFadden G: Apoptosis regulators from DNA viruses. Curr Opin Immunol 10:422–430, 1998
Teodoro JG, Branton PE: Regulation of apoptosis by viral gene products. J Virol 71:1739–1746, 1997
Kaplan D, Sieg S: Role of the Fas/Fas ligand apoptotic pathway in human immunodeficiency virus type 1 disease. J Virol 72:6279–6282, 1998
Berndt C, Mopps B, Angermuller S, Gierschik P, Krammer PH: CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4(+) T cells. Proc Natl Acad Sci USA 95:12556–12561, 1998
Guillerm C, Coudronniere N, Robert-Hebmann V, Devaux C: Delayed human immunodeficiency virus type 1-induced apoptosis in cells expressing truncated forms of CD4. J Virol 72:1754–1761, 1998
Ohnimus H, Heinkelein M, Jassoy C: Apoptotic cell death upon contact of CD4+ T lymphocytes with HIV glycoprotein-expressing cells is mediated by caspases but bypasses CD95 (Fas/Apo-1) and TNF receptor 1. J Immunol 159:5246–5252, 1997
McCloskey TW, Ott M, Tribble E, Khan SA, Teichberg S, Paul MO, et al.: Dual role of HIV Tat in regulation of apoptosis in T cells. J Immunol 158:1014–1019, 1997
Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, et al.: Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31–37, 1994
Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, et al.: Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265:528–530, 1994
Lowin B, Hahne M, Mattmann C, Tschopp J: Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 370:650–652, 1994
Gooding LR, Ranheim TS, Tollefson AE, Aquino L, Duerksen-Hughes P, Horton TM, et al.: The 10,400-and 14,500-dalton proteins encoded by region E3 of adenovirus function together to protect many but not all mouse cell lines against lysis by tumor necrosis factor. J Virol 65:4114–4123, 1991
Shisler J, Yang C, Walter B, Ware CF, Gooding LR: The adenovirus E3–10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J Virol 71:8299–8306, 1997
Ploegh HL: Viral strategies of immune evasion. Science 280:248–253, 1998
Tollefson AE, Hermiston TW, Lichtenstein DL, Colle CF, Tripp RA, Dimitrov T, et al.: Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature 392:726–730, 1998
Li Y, Kang J, Horwitz MS: Interaction of an adenovirus E3 14.7-kilodalton protein with a novel tumor necrosis factor α-inducible cellular protein containing leucine zipper domains. Mol Cell Biol 18:1601–1610, 1998
Li Y, Kang J, Horwitz MS: Interaction of an adenovirus 14.7-kilodalton protein inhibitor of tumor necrosis factor α cytolysis with a new member of the GTPase superfamily of signal transducers. J Virol 71:1576–1582, 1997
Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, et al.: Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517–521, 1997
Hardwick JM: Viral interference with apoptosis. Semin Cell Dev Biol 9:339–949, 1998
Tschopp J, Thome M, Hofmann K, Meinl E: The fight of viruses against apoptosis. Curr Opin Genet Dev 8:82–87, 1998
O'Brien V: Viruses and apoptosis. J Gen Virol 79:1833–1845, 1998
Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y: Kaposi's sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nat Med 3:293–298, 1997
Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A: Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA 90:8479–8483, 1993
Cheng EH, Nicholas J, Bellows DS, Hayward GS, Guo HG, Reitz MS, et al.: A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA 94:690–694, 1997
Zauli G, Gibellini D, Caputo A, Bassini A, Negrini M, Monne M, et al.: The human immunodeficiency virus type-1 Tat protein upregulates bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood 86:3823–3834, 1995
Neil JC, Cameron ER, Baxter EW: p53 and tumour viruses: Catching the guardian off-guard. Trends Microbiol 5:115–120, 1997
Evan G, Littlewood T: A matter of life and cell death. Science 281:1317–1322, 1998
Greenway A, Azad A, McPhee D: Human immunodeficiency virus type 1 Nef protein inhibits activation pathways in peripheral blood mononuclear cells and T-cell lines. J Virol 69:1842–1850, 1995
Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB: Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429–431, 1995
Griffin DE, Hardwick JM: Perspective: virus infections and the death of neurons. Trends Microbiol 7:155–160, 1999
Xu XN, Laffert B, Screaton GR, Kraft M, Wolf D, Kolanus W, et al.: Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor ζ chain. J Exp Med 189:1489–1496, 1999
Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams WV, et al.: HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κβ. Nat Med 3:1117–1123, 1997
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pellegrini, M., Strasser, A. A Portrait of the Bcl-2 Protein Family: Life, Death, and the Whole Picture. J Clin Immunol 19, 365–377 (1999). https://doi.org/10.1023/A:1020598632068
Issue Date:
DOI: https://doi.org/10.1023/A:1020598632068