Abstract
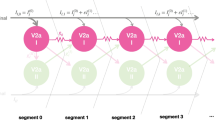
The spinal motor circuits of the Xenopus embryo have been simulated in a 400-neuron network. To explore the consequences of differing patterns of synaptic connectivity within the network for the generation of the motor rhythm, a system of biologically plausible rules was devised to control synapse formation by three parameters. Each neuron had an intrinsic probability of synapse formation (P soma , specified by a space constant λ) that was a monotonically decreasing function of its soma location in the rostro-caudal axis of the simulated network. The neurons had rostral and caudal going axons of specified length (L axon ) associated with a probability of synapse formation (P axon ). The final probability of synapse formation was the product of P soma and P axon . Realistic coordinated activity only occurred when L axon and the probabilities of interconnection were sufficiently high. Increasing the values of the three network parameters reduced the burst duration, cycle period, and rostro-caudal delay and increased the reliability with which the network functioned as measured by the coefficient of variance of these parameters. Whereas both L axon and P axon had powerful and consistent effects on network output, the effects of λ on burst duration and rostro-caudal delay were more variable and depended on the values of the other two parameters. This network model can reproduce the rostro-caudal coordination of swimming without using coupled oscillator theory. The changes in network connectivity and resulting changes in activity explored by the model mimic the development of the motor pattern for swimming in the real embryo.
Similar content being viewed by others
References
Brown P, Dale N (2000a) Adenosine A(1) receptors modulate high voltage-activated Ca2+ currents and motor pattern generation in the Xenopus embryo. J. Physiol. Lond. 525: 655–667.
Brown P, Dale N (2002b) Modulation of K+ currents in Xenopus spinal neurons by p2y receptors: A role for ATP and ADP in motor pattern generation. J. Physiol. 540: 843–850.
Brown P, Dale N (2002c) Spike-independent release of ATP from Xenopus spinal neurons evoked by activation of glutamate receptors. J. Physiol. 543: 851–860.
Buchanan JT (1982) Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: Synaptic interactions and morphology. J. Neurophysiol. 47: 961–975.
Buchanan JT (1992) Neural network simulations of coupled locomotor oscillators in the lamprey spinal cord. Biol. Cybern. 66: 367–374.
Buchanan JT (1993) Electrophysiological properties of identified classes of lamprey spinal neurons. J. Neurophysiol. 70: 2313–2325.
Buchanan JT, Grillner S (1987) Newly identified “glutamate interneurons” and their role in locomotion in the lamprey spinal cord. Science 236: 312–314.
Buchanan JT, Grillner S, Cullheim S, Risling M (1989) Identification of excitatory interneurons contributing to generation of locomotion in lamprey: Structure, pharmacology, and function. J. Neurophysiol. 62: 59–69.
Cohen AH, Holmes PJ, Rand RH (1982) The nature of the coupling between segmental oscillators of the lamprey spinal generator for locomotion:Amathematical model. J. Math. Biol. 13: 345–369.
Dale N (1985) Reciprocal inhibitory interneurones in the Xenopus embryo spinal cord. J. Physiol. 363: 61–70.
Dale N (1993) A large, sustained Na+-dependent and voltage-dependent K+ current in spinal neurons of the frog embryo. J. Physiol.462: 349–372.
Dale N (1995a) Experimentally derived model for the locomotor pattern generator in the Xenopus embryo. J. Physiol. 489: 489–510.
Dale N(1995b) Kinetic characterization of the voltage-gated currents possessed by Xenopus embryo spinal neurons. J. Physiol. 489: 473–488.
Dale N (1998) Delayed production of adenosine underlies temporal modulation of swimming in frog embryo. J. Physiol. 511: 265–272.
Dale N, Gilday D (1996) Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature 383: 259–263.
Dale N, Ottersen OP, Roberts A, Storm-Mathisen J (1986) Inhibitory neurones of a motor pattern generator in Xenopus revealed by antibodies to glycine. Nature 324: 255–257.
Dale N, Roberts A (1985) Dual-component amino-acid-mediated synaptic potentials: Excitatory drive for swimming in Xenopus embryos. J. Physiol. 363: 35–59.
Dale N, Roberts A, Ottersen OP, Storm-Mathisen J (1987) The development of a population of spinal cord neurons and their axonal projections revealed by GABA immunocytochemistry in frog embryos. Proc. R. Soc. Lond. B Biol. Sci. 232: 205–215.
Ermentrout B(1998) The analysis of synaptically generated traveling waves. J. Comput. Neurosci. 5: 191–208.
Ermentrout GB, Kopell N (1984) Frequency plateaus in a chain of weakly coupled oscillators. Siam J. Math. Anal. 15: 215–237.
Hayes BP, Roberts A (1974) The distribution of synapses along the spinal cord of an amphibian embryo: An electron microscope study of junction development. Cell Tiss. Res. 153: 227–244.
Hughes AFW (1959) Studies in embryonic and larval development in Amphibia II: The spinal motor root. J. Embryol. Exp. Morph. 7: 128–145.
Kotaleski JH, Grillner S, Lansner A (1999a) Neural mechanisms potentially contributing to the intersegmental phase lag in lamprey. I. Segmental oscillations dependent on reciprocal inhibition. Biol. Cybern. 81: 317–330.
Kotaleski JH, Lansner A, Grillner S (1999b) Neural mechanisms potentially contributing to the intersegmental phase lag in lamprey. II. Hemisegmental oscillations produced by mutually coupled excitatory neurons. Biol. Cybern. 81: 299–315.
Kopell N, Ermentrout GB (1986) Symmetry and phaselocking in chains of weakly coupled oscillators. Comm.Pure and Appl. Math. 39: 623–660.
Kopell N, Ermentrout GB (1988) Coupled oscillators and the design of central pattern generators. Math. Biosci. 90: 87–109.
Kuenzi FM, Dale N (1998) The pharmacology and roles of two K+ channels in motor pattern generation in the Xenopus embryo. J. Neurosci. 18: 1602–1612.
Li WC, Perrins R, Soffe SR, Yoshida M, Walford A, Roberts A(2001) Defining classes of spinal interneuron and their axonal projections in hatchling Xenopus laevis tadpoles. J. Comp. Neurol. 441: 248–265.
Perrins R, Roberts A (1995a) Cholinergic contribution to excitation in a spinal locomotor central pattern generator in Xenopus embryos. J. Neurophysiol. 73: 1013–1019.
Perrins R, Roberts A (1995b) Cholinergic and electrical synapses between synergistic spinal motoneurons in the Xenopus laevis embryo. J. Physiol. 485: 135–144.
Press WH, Flannery BP, Teukolsky SA, Vetterling WT (1992) Numerical Recipes in C: The Art of Scientific Computing. 2nd edn. Cambridge University Press, Cambridge.
Roberts A, Alford ST (1986) Descending projections and excitation during fictive swimming in Xenopus embryos: Neuroanatomy and lesion experiments. J. Comp. Neurol. 250: 253–261.
Roberts A, Clarke JDW (1982) The neuroanatomy of an amphibian embryo spinal cord. Phil. Trans. R. Soc. B 296: 195–212.
Roberts A, Dale N, Ottersen OP, Storm-Mathisen J (1987) The early development of neurons with GABA immunoreactivity in the CNS of Xenopus laevis embryos. J. Comp. Neurol. 261: 435–449.
Roberts A, Dale N, Ottersen OP, Storm-Mathisen J (1988) Development and characterization of commissural interneurones in the spinal cord of Xenopus laevis embryos revealed by antibodies to glycine. Development 103: 447–461.
Roberts A, Tunstall MJ (1990) Mutual re-excitation with postinhibitory rebound: A simulation study on the mechanisms for locomotor rhythm generation in the spinal cord of Xenopus embryos. Eur. J. Neurosci. 2: 11–23.
Sigvardt KA, Williams TL (1996) Effects of local oscillator frequency on intersegmental coordination in the lamprey locomotor CPG: Theory and experiment. J. Neurophysiol. 76: 4094–4103.
Sillar KT, Roberts A (1991) Segregation of NMDA and non-NMDA receptors at separate synaptic contacts: Evidence from spontaneous EPSPs in Xenopus embryo spinal neurons. Brain Res. 545: 24–32.
Silver RA, Cull-Candy SG, Takahashi T (1996) Non-NMDA glutamate receptor occupancy and open probability at a rat cerebellar synapse with single and multiple release sites. J. Physiol. 494: 231–250.
Soffe SR, Clarke JD, Roberts A (1984) Activity of commissural interneurons in spinal cord of Xenopus embryos. J. Neurophysiol. 51: 1257–1267.
Soffe SR, Zhao FY, Roberts A (2001) Functional projection distances of spinal interneurons mediating reciprocal inhibition during swimming in Xenopus tadpoles. Eur. J. Neurosci. 13: 617–627.
Tunstall MJ, Roberts A (1991) Longitudinal coordination of motor output during swimming in Xenopus embryos. Proc. R. Soc. Lond. B Biol. Sci. 244: 27–32.
Tunstall MJ, Roberts A (1994) A longitudinal gradient of synaptic drive in the spinal cord of Xenopus embryos and its role in coordination of swimming. J. Physiol. 474: 393–405.
van Mier P, Armstrong J, Roberts A (1989) Development of early swimming in Xenopus laevis embryos: Myotomal musculature, its innervation and activation. Neurosci. 32: 113–126.
Wall MJ, Dale N (1993) GABA(B) Receptors modulate glycinergic inhibition and spike threshold in Xenopus embryo spinal neurons. J. Physiol. 469: 275–290.
Wall MJ, Dale N (1995) A alowly activating Ca2+-dependent K+ current that plays a role in termination of swimming in Xenopus embryos. J. Physiol. 487: 557–572.
Williams TL, Sigvardt KA, Kopell N, Ermentrout GB, Remler MP (1990) Forcing of coupled nonlinear oscillators: Studies of intersegmental coordination in the lamprey locomotor central pattern generator. J. Neurophysiol. 64: 862–871.
Yoshida M, Roberts A, Soffe SR (1998) Axon projections of reciprocal inhibitory interneurons in the spinal cord of young Xenopus tadpoles and implications for the pattern of inhibition during swimming and struggling. J. Comp. Neurol. 400: 504–518.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dale, N. Coordinated Motor Activity in Simulated Spinal Networks Emerges from Simple Biologically Plausible Rules of Connectivity. J Comput Neurosci 14, 55–70 (2003). https://doi.org/10.1023/A:1021176301776
Issue Date:
DOI: https://doi.org/10.1023/A:1021176301776