Abstract
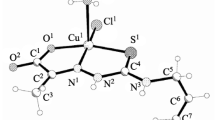
A tetraazamacrocyclic ligand, L, containing six non-equivalent benzene rings, derived from the condensation of benzil with 1,2- diaminobenzene, has been isolated and its complexes [MLCl2] (M = Ni2+ and Cu2+) prepared and characterized by elemental analysis, i.r., u.v.–vis., e.p.r. spectral studies, magnetic moments, redox potentials and conductivity measurements. The complexes have axially elongated octahedral geometries with two axial chlorines, and adopt the trans-configuration. These studies also indicate the covalent nature and the high-spin octahedral structure for these complexes. A cyclic voltammetric investigation reveals that the complexes exhibit a single one-electron redox couple, as anticipated for a copper(II) complex (Cu2+/Cu+) and a single two-electron redox couple for a nickel(II) complex (Ni2+/Ni0). The electrochemical processes are considered quasi-reversible. Antimicrobial activities of the ligand and the complexes have been tested against Bacillus megaterium and Candida tropicallis.
Similar content being viewed by others
References
K.Y. Choi, K.M. Chun and I.H. Suh, Inorg. Chem. Commun., 2, 210 (1999).
E.M., Martin, R.D. Bereman and P. Singh, Inorg. Chim. Acta, 30, 957 (1991).
E.M. Martin, R.D. Bereman and J. Dorfman, Inorg. Chim. Acta, 176, 247 (1990).
D.E. Fenton, U. Casellato, P.A. Vigato and M. Vidali, Inorg. Chim. Acta, 95, 187 (1984).
N. Kitajima and Y. Morooko, Chem. Rev., 94, 737 (1994).
L. Casella and L. Rigoni, J. Chem. Soc., Chem. Commun., 1668 (1985).
G. Albertin, E. Bordignon and A.A. Orio, Inorg. Chem., 14, 1411 (1975).
B.S. Garg and L. Kapur, Inorg. Chim. Acta, 173, 223 (1990).
W. Zishen, L. Zhiping and Y. Zhenhuan, Transition Met. Chem., 18, 291 (1993).
A.K. Usha and S. Chandra, Synth. React. Inorg. Met. Org. Chem., 22, 1565 (1992).
R.W. Hay, B. Jeragh, G. Ferguson, B. Kaitner and B.L. Ruhl, J Chem. Soc., Dalton Trans., 1531 (1982).
T. Ito, M. Kato and H. Ito, Bull. Chem. Soc. Jpn., 57, 2641 (1984).
R. Vicente, A. Escuer, J. Ribas and X. Solans. Inorg. Chem., 31, 1726 (1992).
A. Escuer, R. Vicente, J. Ribas, M.S. El Fallah and X. Solans, Inorg. Chem., 32, 1033 (1993).
R. Vicente, A. Escuer, J. Ribas, M.S. El Fallah, X. Solans and M.J. Font-Bardia, Inorg. Chem., 34, 1278 (1995).
A. Escuer, R. Vicente, M.S. El Fallah, X. Solans and M.J. Font-Bardia. J. Chem. Soc., Dalton Trans., 1013 (1996).
K.Y. Choi, M.R. Oh and I.H. Suh, Chem. Lett., 147 (1997).
K.Y. Choi, H. Ryu and I.H. Suh, Polyhedron, 17, 1241 (1998).
R.S. Sharma and W.T. Mehrotra, J. Ind. Council Chem., 17, 43 (2000).
W.P Singh and V.N. Singh, Synth. React. Inorg. Met. Org. Chem., 27, 695 (1997).
K. Mochizuki and T. Kando, Inorg. Chem., 34, 6241 (1995).
K.Y. Choi, S.N. Choi and I.H. Suh, Polyhedron, 17, 1415 (1998).
R. Vicente, A. Escuer, M.S. El Fallah, X. Solans and M.J. Font-Bardia, Inorg. Chim. Acta, 261, 227 (1997).
D.D. Perrin, W.L.F. Armarego and D.R. Perrin, Purification of Laboratory Chemicals. 2nd edn., Pergamon Press, New York, 1985.
C.H. Collins, P.M. Lyne and J.M. Grange, Microbiological Methods, 3rd edit., Butterworth & Co., 1989, p. 410.
K.R. Koch, J. Coord. Chem., 22, 289 (1991).
W.J. Geary, Coord. Chem. Rev., 7, 81 (1971).
S.G. Kang, J.K. Kweon and S.K. Jung, Bull. Korean Chem. Soc., 12, 483(1991).
K.Y. Choi, Y.J. Kim, H. Ryu and I.H. Suh, Inorg. Chem. Commun., 2, 176 (1999).
B.N. Figgis, Introduction to Ligand Fields. Wiley, New Delhi, 1966.
R. Ruiz, J. Sanz, B. Cervera, F. Lloret, M. Julve, C. Bois, J. Faus, and M.C. Munoz. J. Chem. Soc., Dalton Trans., 1623 (1993).
A.L. Nivorozhkin, H. Toflund, P.L. Jorgensen and L.E. Nivorozhkin, J. Chem, Soc., Dalton Trans., 1215 (1996).
G.S. Patterson and R.H. Holm, Bioinorg. Chem., 4, 257 (1975).
L. Aronne, B.C. Dunn, J.R. Vyvyan, C.W. Souvignier, M.J. Mayer, T.A. Harwood, C.A. Salhi, S.N. Goldie, L.A. Ochrymowycz and B.D. Rorabacher, Inorg. Chem., 34, 357 (1995).
A.J. Bard and L.R. Faukner, in Masson, (ed.) Electrochemistry, Adaptation Francaise, Paris, 1983, p 239.
W.R. McWhinne, J. Inorg. Nucl. Chem., 27, 1063 (1965).
K.K. Narang, J.P. Pandey and V.P. Singh, Polyhedron, 13, 529 (1994).
W.E. Blumberg and Y. Peisach, J. Biol Chem., 240, 870 (1965).
Y. Gok and S. Karabocek, J. Coord. Chem., 40, 203 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aqra, F.M. Encapsulation of nickel(II) and copper(II) by a tetraazamacrocyclic ligand. Transition Metal Chemistry 28, 224–228 (2003). https://doi.org/10.1023/A:1022975829744
Issue Date:
DOI: https://doi.org/10.1023/A:1022975829744